US6044843A - Moisture resistant airway adapter for monitoring constituent gases - Google Patents
Moisture resistant airway adapter for monitoring constituent gases Download PDFInfo
- Publication number
- US6044843A US6044843A US08/863,637 US86363797A US6044843A US 6044843 A US6044843 A US 6044843A US 86363797 A US86363797 A US 86363797A US 6044843 A US6044843 A US 6044843A
- Authority
- US
- United States
- Prior art keywords
- airway adapter
- contact angle
- gas
- adapter
- window
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000007789 gas Substances 0.000 title claims abstract description 109
- 239000000470 constituent Substances 0.000 title claims abstract description 31
- 238000012544 monitoring process Methods 0.000 title description 10
- 230000000241 respiratory effect Effects 0.000 claims abstract description 37
- 238000009833 condensation Methods 0.000 claims abstract description 26
- 230000005494 condensation Effects 0.000 claims abstract description 26
- 238000005259 measurement Methods 0.000 claims abstract description 20
- 230000002209 hydrophobic effect Effects 0.000 claims abstract description 16
- 238000000576 coating method Methods 0.000 claims description 19
- 239000011248 coating agent Substances 0.000 claims description 18
- 230000003287 optical effect Effects 0.000 claims description 15
- 238000002835 absorbance Methods 0.000 claims description 12
- 238000000034 method Methods 0.000 claims description 12
- 239000000463 material Substances 0.000 claims description 11
- 229920000642 polymer Polymers 0.000 claims description 11
- 150000001875 compounds Chemical class 0.000 claims description 4
- 230000008569 process Effects 0.000 claims description 4
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical group O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 claims description 4
- BFKJFAAPBSQJPD-UHFFFAOYSA-N tetrafluoroethene Chemical group FC(F)=C(F)F BFKJFAAPBSQJPD-UHFFFAOYSA-N 0.000 claims description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 3
- 229920000669 heparin Polymers 0.000 claims description 3
- 229960002897 heparin Drugs 0.000 claims description 3
- 229910052760 oxygen Inorganic materials 0.000 claims description 3
- 239000001301 oxygen Substances 0.000 claims description 3
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 3
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 3
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 claims description 2
- DNCYBUMDUBHIJZ-UHFFFAOYSA-N 1h-pyrimidin-6-one Chemical group O=C1C=CN=CN1 DNCYBUMDUBHIJZ-UHFFFAOYSA-N 0.000 claims description 2
- 239000004812 Fluorinated ethylene propylene Substances 0.000 claims description 2
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 claims description 2
- 239000002202 Polyethylene glycol Substances 0.000 claims description 2
- 230000015572 biosynthetic process Effects 0.000 claims description 2
- 229920001577 copolymer Polymers 0.000 claims description 2
- 238000010168 coupling process Methods 0.000 claims description 2
- 229920002313 fluoropolymer Polymers 0.000 claims description 2
- 229920002674 hyaluronan Polymers 0.000 claims description 2
- 229960003160 hyaluronic acid Drugs 0.000 claims description 2
- 238000006385 ozonation reaction Methods 0.000 claims description 2
- 229920009441 perflouroethylene propylene Polymers 0.000 claims description 2
- 229920002401 polyacrylamide Polymers 0.000 claims description 2
- 229920001223 polyethylene glycol Polymers 0.000 claims description 2
- 238000006116 polymerization reaction Methods 0.000 claims description 2
- 229920001296 polysiloxane Polymers 0.000 claims description 2
- 239000011347 resin Substances 0.000 claims description 2
- 229920005989 resin Polymers 0.000 claims description 2
- 125000005523 4-oxopentanoic acid group Chemical group 0.000 claims 1
- 239000008119 colloidal silica Substances 0.000 claims 1
- 239000011527 polyurethane coating Substances 0.000 claims 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 24
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 22
- 229910002092 carbon dioxide Inorganic materials 0.000 description 13
- 239000001569 carbon dioxide Substances 0.000 description 13
- 239000007788 liquid Substances 0.000 description 11
- 230000006835 compression Effects 0.000 description 8
- 238000007906 compression Methods 0.000 description 8
- 230000029058 respiratory gaseous exchange Effects 0.000 description 8
- 230000005660 hydrophilic surface Effects 0.000 description 7
- 230000005661 hydrophobic surface Effects 0.000 description 7
- -1 polypropylene Polymers 0.000 description 6
- 229920003023 plastic Polymers 0.000 description 5
- 239000004033 plastic Substances 0.000 description 5
- 230000005855 radiation Effects 0.000 description 5
- 229920000728 polyester Polymers 0.000 description 4
- 238000005070 sampling Methods 0.000 description 4
- 229910052594 sapphire Inorganic materials 0.000 description 4
- 239000010980 sapphire Substances 0.000 description 4
- 206010002091 Anaesthesia Diseases 0.000 description 3
- 239000004698 Polyethylene Substances 0.000 description 3
- 239000004743 Polypropylene Substances 0.000 description 3
- 230000037005 anaesthesia Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 229920000573 polyethylene Polymers 0.000 description 3
- 229920001155 polypropylene Polymers 0.000 description 3
- JOOXCMJARBKPKM-UHFFFAOYSA-N 4-oxopentanoic acid Chemical compound CC(=O)CCC(O)=O JOOXCMJARBKPKM-UHFFFAOYSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 239000004809 Teflon Substances 0.000 description 2
- 229920006362 Teflon® Polymers 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000002238 attenuated effect Effects 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 230000005670 electromagnetic radiation Effects 0.000 description 2
- 239000003193 general anesthetic agent Substances 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 238000007726 management method Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 238000003825 pressing Methods 0.000 description 2
- 206010006458 Bronchitis chronic Diseases 0.000 description 1
- 206010014561 Emphysema Diseases 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- 208000010496 Heart Arrest Diseases 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 230000003444 anaesthetic effect Effects 0.000 description 1
- 239000003994 anesthetic gas Substances 0.000 description 1
- 208000008784 apnea Diseases 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 206010006451 bronchitis Diseases 0.000 description 1
- 230000002612 cardiopulmonary effect Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 208000007451 chronic bronchitis Diseases 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000012864 cross contamination Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 229960003537 desflurane Drugs 0.000 description 1
- DPYMFVXJLLWWEU-UHFFFAOYSA-N desflurane Chemical compound FC(F)OC(F)C(F)(F)F DPYMFVXJLLWWEU-UHFFFAOYSA-N 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 229960000305 enflurane Drugs 0.000 description 1
- JPGQOUSTVILISH-UHFFFAOYSA-N enflurane Chemical compound FC(F)OC(F)(F)C(F)Cl JPGQOUSTVILISH-UHFFFAOYSA-N 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000004868 gas analysis Methods 0.000 description 1
- 229960003132 halothane Drugs 0.000 description 1
- BCQZXOMGPXTTIC-UHFFFAOYSA-N halothane Chemical compound FC(F)(F)C(Cl)Br BCQZXOMGPXTTIC-UHFFFAOYSA-N 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 229940040102 levulinic acid Drugs 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229920002492 poly(sulfone) Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229960002078 sevoflurane Drugs 0.000 description 1
- DFEYYRMXOJXZRJ-UHFFFAOYSA-N sevoflurane Chemical compound FCOC(C(F)(F)F)C(F)(F)F DFEYYRMXOJXZRJ-UHFFFAOYSA-N 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- 238000001771 vacuum deposition Methods 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- 230000003519 ventilatory effect Effects 0.000 description 1
- 230000037303 wrinkles Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/08—Bellows; Connecting tubes ; Water traps; Patient circuits
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/08—Bellows; Connecting tubes ; Water traps; Patient circuits
- A61M16/0816—Joints or connectors
- A61M16/0841—Joints or connectors for sampling
- A61M16/085—Gas sampling
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/75—General characteristics of the apparatus with filters
- A61M2205/7527—General characteristics of the apparatus with filters liquophilic, hydrophilic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/75—General characteristics of the apparatus with filters
- A61M2205/7536—General characteristics of the apparatus with filters allowing gas passage, but preventing liquid passage, e.g. liquophobic, hydrophobic, water-repellent membranes
Definitions
- the present invention relates generally to airway adapters used with gas analyzers for monitoring constituent respiratory gases in a patient.
- the present invention relates particularly to an airway adapter with a surface confronting the airway which interacts with water in the patient's breath so as to reduce the impact of water on the monitoring of the constituent gases.
- monitoring respiration is a very reliable method of determining the condition of a patient and, moreover, is one which provides an instant indication of adverse conditions as compared with other life signs which take longer periods to depart from normal values.
- Such an immediate indication is of great interest in connection with patients undergoing surgery or who are in other life-threatening situations, and in connection with monitoring infants who are subject to cessation of breathing (apnea) for no apparent cause.
- Respiratory gas analyzers function by passing electromagnetic radiation (typically light) of a specific wavelength (typically infrared) through the respiratory gas and measuring the absorption for a component being monitored (such as CO 2 ).
- electromagnetic radiation typically light
- a specific wavelength typically infrared
- One method of monitoring breathing which has been used extensively involves monitoring the difference in carbon dioxide (CO 2 ) content between an individual's inspired and expired gas streams. It is impossible to breathe without the carbon dioxide content of the expired gas stream varying from that of the inspired gas stream by at least about 2%.
- Capnography the measurement of carbon dioxide levels in the airway, is one particular monitoring technology that aids clinicians in critical patient management decisions. Capnography assists clinicians in patient management decisions by providing the means to assess a large number of problems related to ventilation, cardiopulmonary functions and metabolism. Capnography can help clinicians monitor the integrity of gas delivery systems and mechanical ventilators as well. It can provide early warning of events which may indicate an obstruction of the patient's airway tube or disconnection of the ventilatory circuit, the onset of pulmonary disease or changes in physiologic status. In addition, waveforms displayed on a monitor corresponding to carbon dioxide concentrations may be used by clinicians to assess emphysema, asthma, chronic bronchitis, pneumonia and cardiac arrest.
- Most capnometers are comprised of an airway adapter (sometimes referred to as a cuvette), an emitter, a detector, and a processor.
- the constituent gas flows through the airway adapter and between the emitter and detector, which are placed behind windows on either side of the airway adapter. Measurement is made directly through the windows.
- the detector receives the energy that is transmitted by the emitter that is not absorbed by the constituent gas, and the processor processes the signal from the detector. For example, emitted infrared light of a selected wavelength band is attenuated in correspondence to the quantity of carbon dioxide in the respiratory gas.
- U.S. Pat. No. 4,648,396 discloses a respiration detector which features an infrared source and detector pair disposed on opposite sides of a cuvette through which the gas stream is inhaled and exhaled by a patient.
- U.S. Pat. No. 5,067,492 discloses a disposable endotracheal airway adapter that connects in series with a respirator or anesthesia breathing circuit and allows the passage of infrared radiation through the gases for measuring the constituent concentration of respiratory gases.
- U.S. Pat. No. 5,092,342 discloses a sensor arrangement including a housing containing a transmitter, a receiver, and a heatable holder for a measuring cuvette.
- U.S. Pat. No. 5,146,092 discloses an airway adapter with a heater provided to heat the casing of the airway adapter associated with a transducer to a temperature high enough to keep condensation from forming on the windows.
- Heating the cuvette has a number of disadvantages.
- the present invention provides an airway adapter with a window having a surface confronting the airway that controls the condensation formed on the window in the airway adapter.
- the surface of the window is sufficiently hydrophilic to cause the condensation on the window to be drawn out to form a layer of moisture so thin that it causes little interference with the gas analyzer measurements conducted through the windows.
- the surface of the window is sufficiently hydrophobic to cause the condensation to form in beads on the window so that the areas between the beads are sufficiently free from condensation so that gas analyzer measurements can be made through those areas.
- the surface of the window can be both hydrophilic and hydrophobic to provide the advantages set forth herein.
- an airway adapter for use with a respiratory gas analyzer comprising a tubular portion having a window positioned whereby respiratory gases passing through the airway adapter flow past the window and being adapted to receive an emitter and a receiver for measuring through the window energy absorbance of a constituent gas in the respiratory gases, the window comprises at least one surface which interacts with moisture in the respiratory gases so as to reduce the interference of moisture with the measurement of the constituent gases by the gas analyzer.
- the surface of the windows can be hydrophobic, hydrophilic, or a combination thereof.
- an airway adapter for use with a respiratory gas analyzer having a tubular portion having a film window positioned in a seat having a seating surface and a receiving surface.
- a securing member is pressed into the seat toward the seating surface to hold the film window taut so as to provide uniform optical transmission for measuring the optical absorbance of the constituent gas.
- FIG. 1 is a perspective view of one embodiment of an airway adapter for use with a gas sensor and analyzer in accordance with the present invention
- FIG. 2 is an exploded view of the gas sensor shown in FIG. 1;
- FIG. 3 is a perspective view of another embodiment of the airway adapter of the present invention shown rotated 90E counter-clockwise such that the right side of the airway adapter is facing upward;
- FIG. 3A is a right side view of the airway adapter shown in FIG. 3 rotated to its normal orientation
- FIG. 4 is a top plan view of the airway adapter shown in FIG. 3A;
- FIG. 5 is a cross-sectional view of the airway adapter in FIG. 3A taken along line 5--5;
- FIG. 6 is a cross-sectional view of the airway adapter in FIG. 3A taken along line 6--6;
- FIG. 7 is a cross-sectional view of the airway adapter in FIG. 4 taken along line 7--7;
- FIG. 8 is an enlarged view of one embodiment of the windows in accordance with the present invention.
- FIG. 9 is an enlarged view of another embodiment of the windows in accordance with the present invention.
- FIG. 10 is a side view of another embodiment of the airway adapter of the present invention.
- FIG. 11 is a top view of the airway adapter of FIG. 10;
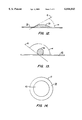
- FIG. 12 is a schematic of a sessile drop contact angle for a hydrophilic surface
- FIG. 13 is a schematic of a sessile drop contact angle for a hydrophobic surface
- FIG. 14 is one embodiment of the windows in accordance with the present invention comprising a combination hydrophilic and hydrophobic surface.
- an airway adapter 4 comprising a cuvette 2 which is used to prevent the patient respiratory gases being monitored from coming in contact with a respiratory gas analyzer sensor 3 which connects to gas analyzer 1.
- the gases being monitored can be carbon dioxide, oxygen, ethanol, metabolic trace gases such as acetone or anesthetic gases such as isoflurane, halothane, desflurane, sevoflurane and enflurane.
- the cuvette or conduit 2 is designed to connect in series with tubing used to connect a patient to a mechanical ventilator or anesthesia breathing circuit.
- the cuvette or conduit is not limited to being transparent.
- the airway adapter 4 is preferably very light weight so that it does not interfere with the breathing tube which is usually inserted into a patient's trachea.
- the cuvette or conduit 2 of the present invention provides an optical path through which the gas sensor 3 can pass electromagnetic radiation onto a sample of respiratory gas(es) for analysis.
- the airway adapter 4 can be sterilized for re-use or disposed of after one use. Such single patient use saves sterilization expenses and eliminates cross-contamination. This is especially useful with highly contagious diseases such as tuberculosis, but is also a benefit in routine cases where an unknown disease may be carried but not active. Also, since the airway adapter 4 of the present invention is relatively small and light weight, it may be placed close to the patient's mouth so as to allow quantitative readings for purposes of determining the concentrations of the constituents in the expired gas.
- the airway adapter 4 is configured to fit into the housing 5 of a gas sensor 3.
- a gas sensor 3 that may be used with the airway adapter 4 of the present invention is shown in FIGS. 1 and 2.
- Portions 7,9 of the gas sensor housing 5 extend down over a center section 19 of the cuvette or conduit 2 so as to accurately locate an infrared photo-emitter 11 and photo-detector 13 over windows 17 so that the photo-emitter and photo-detector are at a predetermined distance from each other.
- Housing member 6 is assembled with the housing 5 to enclose the photo-emitter 11 and photo-detector 13.
- An example of a commercially available gas sensor is Model N-6000 available from Nellcor, Inc., Pleasanton, Calif., 94588, the assignee of the present invention.
- the photo-emitter 11 is preferably an infrared light source, such as the incandescent broad band lamp, Model OL-3070, manufactured by Oshino Lamps (5 volt, 1.6 amp, 2-1, 1/8 in. bulb).
- the photo-detector 13 contains an infrared detector and amplifier electronics. Signal connections are made via a cable 15 to gas analyzer 1.
- the center section 19 of airway adapter 4 comprises a truncated cylinder with generally parallel sides 20,22.
- the airway adapter 4 also has two cylindrical end sections 21,23 with a sampling passage 25 extending from end-to-end through the adapter 4.
- the end sections 21,23 are axially aligned with center section 19.
- the central section 19 of the cuvette 2 provides a seat for the gas sensor 3.
- FIGS. 3-7 show another embodiment of the airway adapter 4 in accordance with the present invention.
- the cylindrical end sections 21,23 are sufficiently tapered so as to form standard conical fittings for attachment to the tubing used to connect a patient to a mechanical ventilator or anesthesia breathing circuit.
- U-shaped flange 8 can be provided in some embodiments to help locate and maintain gas sensor 3 in the proper location on airway adapter 4.
- Protrusion 12 can also be provided to engage a detent on the gas sensor to help maintain the location of the gas sensor.
- Adapter 4 is preferably made from polypropylene, polyethylene, polysulfone or a comparable polymer.
- openings or ports 27,28 are formed in the generally parallel sides 20,22 in the center section 19 of the cuvette 2.
- the openings or ports 27,28 are aligned and spaced in a predetermined widthwise configuration.
- FIG. 5 shows a cross-sectional view taken along line 5--5 in FIG. 3A of the airway adapter 4.
- the openings 27,28 define a predetermined path length distance identified by reference character L from each other.
- the optical path L extends from the infrared photo-emitter 11 in gas sensor 3 transversely across the cuvette or conduit 2 and the gas(es) flowing therethrough to the photo-detector 13.
- the airway adapter 4 is designed such that it conforms to the housing 5 of the gas sensor 3 so that a precision optical path length may be maintained even when less expensive materials such as plastic are used for forming the airway adapter.
- the openings or ports 27,28 are sealed by windows 17 to keep the gas(es) flowing through the conduit 2 from escaping through openings 27,28 and to keep foreign material from entering the sampling passage 25 of the conduit 2. Perhaps even more importantly, these windows help to define the length of the optical path L for the radiation traversing the conduit 2. This is important because the radiation is attenuated to an extent proportional to the number of molecules of the designated constituent gas between the windows 17 and in that volume circumscribed by the windows. Consequently, even small volume variations can significantly affect the accuracy of a signal ultimately produced indicating the concentration of a designated constituent gas.
- the infrared photo-emitter 11 and photo-detector 13 can be located on the same side of cuvette or conduit 2 with a mirror on the other side of conduit 2 to reflect the radiation emitted from the photo-emitter back to the photo-detector.
- gas sensor 3 can be employed to measure the concentration of a designated gas(es) flowing through the sampling passage 25 in the airway adapter 4.
- moisture principally water vapor
- the condensation or fog is created when warm, moist gas(es) inside the respiratory circuit come(s) in contact with the cooler windows 17 of the airway adapter 4. The result may be a degradation in performance and loss of accuracy.
- the resulting condensation or fog absorbs some unknown portion of the infrared energy at the wavelengths used to measure carbon dioxide, anesthetic agents and other gas(es).
- This problem is solved simply and elegantly in accordance with the present invention by using windows having surfaces that interact in a desired way with the moisture or water that condenses from the patient's breath so as to reduce the impact of the moisture on the monitoring of the constituent gases by the gas sensor 3.
- the window surface itself or a coating on the window having characteristics described herein, can be used in accordance with the present invention.
- the window surface or coating is sufficiently hydrophilic so as to wick away the condensation and form a water or moisture layer over the surface of the windows 17, which layer is sufficiently thin and uniform to enable accurate measurements to be conducted therethrough.
- the attenuation caused by the uniform thin layer of moisture is reduced to an acceptable level in the desired wavelengths.
- the attenuation resulting from a particular surface or coating is thereby uniform and consistent allowing compensation to be made and accuracy of measurement maintained.
- the hydrophilic surface or coating preferably should have 50% or less optical attenuation in the desired wavelengths.
- the desired wavelengths are at or above 3 microns for detecting carbon dioxide.
- the desired wavelength may be anywhere between ultraviolet and infrared (i.e., 300 nm to 20,000 nm).
- a common measurement of the relative hydrophilicity of surfaces is the sessile drop contact angle " (FIG. 12), which is defined as that angle formed in the interior of the drop between surface 38 on which drop 39 rests and line 41 tangential to the drop at its point of contact with the surface.
- a surface is said to become more hydrophilic if the contact angle of the sessile drop is reduced.
- the work of adhesion between the polymer surface and liquid water be equal to or less than the work of cohesion of the liquid water itself. Under this condition, the liquid water can spread indefinitely over the surface, since energetically the system is indifferent to whether the water is in contact with the polymer or with itself.
- Contact angle is readily measured by placing a drop of pure water (approximately 0.05 ml) on a clean and level surface. The drop is then imaged from the side with an optical microscope C or an image of the drop is projected onto a screen C and the contact angle is measured geometrically. The contact angle can also be measured using the Wilhelmi Plate Method of immersing a plate with a known surface area into a liquid with a known surface tension and measuring the force required to remove the plate.
- Uncoated plastics such as polycarbonate, polyethylene, polypropylene, and polyester have contact angles of about 84E, 89E, 93E, and 90E respectively.
- some polymers may be inherently hydrophilic to some degree and have contact angles greater than 50E.
- Sapphire has a contact angle of about 90E. If the contact angle is greater than about 50E, as may occur for example in polymers that are neither coated nor have any other type of hydrophilic surface modification, the surface is insufficiently hydrophilic for this embodiment of the invention.
- the hydrophilic surface produces a sessile drop contact angle of about 50E or less, more preferably about 20E or less, and most preferably about 10E or less.
- the particularly preferred surface has a contact angle in the range of about 2.5E to about 5E for optimum performance.
- Polymeric materials such as polyurethane coated polyester exhibit a high degree of hydrophilicity C sessile drop contact angle of about 19E C and can be used with the present invention.
- Other materials such as "VISTEX,” a polyester terephthalate and aliphatic urethane interpolymer with polyvinyl pyrrolidone which is preferred, (available from Film Specialties, Inc., Whitehouse, N.J.), or polyacrylamide, polyvinylpyrrolidone, polyethylene glycol, hyaluronic acid, heparin, and polyacrylamide-heparin complexes can be used also.
- a surface coating it can be applied to the surface of windows 17 as a film or liquid coating or with a photochemically coupling process, a copolymer formation process, a plasma polymerization process, vacuum deposition process or an ozonation technique.
- the surface of the windows 17 or the coating can be hydrophobic.
- the hydrophobicity causes the condensation to form droplets.
- the droplets form interstices between the droplets that are substantially clear and dry such that sufficient energy at the desired wavelengths traverses the airway adapter through windows 17 for accurate measurements.
- the hydrophobic surface or coating preferably should have 50% or less optical attenuation in the desired wavelengths.
- the desired wavelengths are at or above 3 microns for detecting carbon dioxide.
- the desired wavelength may be anywhere between ultraviolet and infrared (i.e., 300 nm to 20,000 nm).
- the sessile drop contact angle " (FIG. 13), which is defined as that angle formed in the interior of the drop between surface 43 on which drop 45 rests and line 41 tangent to the drop at its point of contact with the surface.
- a surface is said to become more hydrophobic if the contact angle of the sessile drop is increased.
- the work of adhesion between the polymer surface and liquid water must be greater than the work of cohesion of the liquid water itself. Under this condition, liquid water cannot spread indefinitely over such a surface, it must form compact drops since energetically the system is sensitive to whether the water is in contact with the surface or with itself. Therefore, the liquid water can form substantial droplets spaced across the surface.
- Contact angle " is readily measured as discussed previously.
- Some materials may be inherently hydrophobic to some degree. If the contact angle is 93E or less, as occurs for example on materials that are neither coated nor have any other type of hydrophobic surface modification such as polycarbonate, polyethylene, polypropylene and polyester mentioned above, the surface is insufficiently hydrophobic for this embodiment of the invention.
- the hydrophobic surface produces a sessile drop contact angle of 94E or greater, more preferably about 100E or greater, and most preferably about 120E or greater.
- a particularly preferred surface has a contact angle of about 160E or greater for optimum performance.
- Materials that exhibit strong hydrophobicity and can be used in accordance with the present invention include polymers such as tetrafluoroethylene fluorocarbon polymers C sessile drop contact angle of about 105E C and fluorinated ethylene-propylene resins sold under the trade names "TEFLON AF” and “TEFLON PFA” (available from E. I. Du Pont de Nemours, Co., Wilmington, Del.) and silicone resin-colloidal silica complexes such as the one sold under the trade name "SILVUE XF-094" (available from SDC Coatings, Inc., Anaheim, Calif.).
- polymers such as tetrafluoroethylene fluorocarbon polymers C sessile drop contact angle of about 105E C and fluorinated ethylene-propylene resins sold under the trade names "TEFLON AF” and “TEFLON PFA” (available from E. I. Du Pont de Nemours, Co., Wilmington, Del.) and silicone resin-colloidal si
- FIG. 8 shows a close-up view of one embodiment of the windows 17 of the present invention in which the window 17 is a polymeric material, preferably a film, which inherently has surface characteristics as described herein or has its surface coated with a material having surface characteristics described herein. Such materials are preferably used since they are inexpensive, transparent to infrared energy and relatively rigid, yet are not susceptible to moisture condensation.
- An annular seat 31 is provided in each port 27,28 for receiving the windows 17.
- the polymeric window 17 is pressed into the annular seat 31 and held in place by friction press fit with the compression ring 29.
- window 17 is placed over each port 27,28. Then the compression ring is used to stretch window 17 taut as the compression ring is pressed into seat 31.
- the window 17 has a diameter slightly larger than the diameter of annular seat 31 and the diameter of the compression ring. In this way, window 17 is secured to airway adapter 4 along receiving surface 30.
- the window goes from a relaxed state to a stretched state in order to hold the optical path length L constant. It is important that the windows do not have any wrinkles, waves, deformations, etc. because even small variations in the optical path can significantly affect the accuracy of an ultimately produced, concentration indicative signal.
- the seated position of the window is shown as 17N and of the compression ring as 29N.
- Compression ring 29 can be plastic or metallic.
- the plastic or i metallic compression ring can be used in conjunction with a heater to heat the window and rid the window of moisture whether the window is hydrophobic, hydrophilic or neither.
- Plastic can be used to achieve the desired amount of heat conduction to accomplish the drying function because the ring is small and the amount of heat to be supplied is small.
- the polymeric window 17 can be held in place by heat pressing the window periphery around the annular seat, by heat staking or by adhering the window to the adapter body by some other acceptable adhering technique such as ultrasonic bonding or by using a suitable adhesive.
- sapphire windows with either a hydrophobic or hydrophilic surface film, coating or treatment as described herein can be used with the present invention because they absorb very little infrared radiation in the desired wavelengths.
- FIG. 9 shows a close-up view of such an embodiment.
- the sapphire window 17 is pressed into the annular seat 31 and held in place by a friction press fit or with a suitable adhesive. The seated position of the window is shown as 17N.
- the sapphire window can also be held in place by heat pressing the window periphery around the annular seat, by heat staking, or by ultrasonic bonding.
- FIGS. 10 and 11 show another embodiment of the airway adapter of the present invention.
- the airway adapter 4N is used for patients who are not intubated with an endotracheal tube and must be monitored with a nasal cannula.
- the nasal cannula is a flexible tube which is placed slightly within the nostrils.
- a pump continuously withdraws gas from the nasal cannula through the airway adapter 4N.
- the airway adapter 4N is fitted with the same windows 17 in the same optical arrangement as the airway adapters previously described.
- FIG. 11 shows that the inlet 33 and outlet 35 are placed off center in order to ensure that the constituent gas(es) passing through the sampling chamber 37 are well mixed for accurate analysis.
- the coating or surface of the windows 17 can also be a combination of hydrophilic and hydrophobic properties.
- FIG. 14 illustrates one embodiment of window 17 with a hydrophobic surface 43 surrounded by a hydrophilic surface 38.
- the hydrophilic portion can wick the condensation away from the hydrophobic portion thus minimizing the amount of water collected on the hydrophobic area.
- the hydrophobic portion can urge the condensation to the hydrophilic portion.
- Various patterns of alternating hydrophobic and hydrophilic areas can be used, such as strips, dots, checks, concentric rings, etc., to control the condensation on the surface so that the desired accuracy of measurements can be achieved.
- the entire or a portion of the inside and/or outside of the airway adapter can be hydrophilic, hydrophobic, or a combination thereof.
Landscapes
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Pulmonology (AREA)
- Engineering & Computer Science (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Investigating Or Analysing Materials By Optical Means (AREA)
- Investigating Or Analysing Biological Materials (AREA)
Abstract
Description
Claims (24)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/863,637 US6044843A (en) | 1997-05-28 | 1997-05-28 | Moisture resistant airway adapter for monitoring constituent gases |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/863,637 US6044843A (en) | 1997-05-28 | 1997-05-28 | Moisture resistant airway adapter for monitoring constituent gases |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US6044843A true US6044843A (en) | 2000-04-04 |
Family
ID=25341459
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/863,637 Expired - Lifetime US6044843A (en) | 1997-05-28 | 1997-05-28 | Moisture resistant airway adapter for monitoring constituent gases |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US6044843A (en) |
Cited By (61)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6190327B1 (en) * | 1999-05-05 | 2001-02-20 | Nonin Medical, Inc. | Disposable airway adapter for use with a carbon dioxide detector |
| US6258040B1 (en) * | 1998-09-16 | 2001-07-10 | Nihon Kohden Corporation | Airway adapter for non-dispersive infrared gas analyzer |
| US6277645B1 (en) | 1998-08-03 | 2001-08-21 | James R. Mault | Method and apparatus for respiratory gas analysis employing measurement of expired gas mass |
| US6309360B1 (en) | 1997-03-17 | 2001-10-30 | James R. Mault | Respiratory calorimeter |
| EP1112716A3 (en) * | 1999-12-31 | 2001-11-07 | GE Marquette Medical Systems, Inc. | Low cost main stream gas analyzer system |
| US20020047867A1 (en) * | 2000-09-07 | 2002-04-25 | Mault James R | Image based diet logging |
| US20020055857A1 (en) * | 2000-10-31 | 2002-05-09 | Mault James R. | Method of assisting individuals in lifestyle control programs conducive to good health |
| US6402698B1 (en) | 1998-02-05 | 2002-06-11 | James R. Mault | Metabolic calorimeter employing respiratory gas analysis |
| US6406435B1 (en) | 1998-11-17 | 2002-06-18 | James R. Mault | Method and apparatus for the non-invasive determination of cardiac output |
| US20020104536A1 (en) * | 2000-09-28 | 2002-08-08 | Richey Joseph B. | Carbon dioxide-based Bi-level CPAP Control |
| US20020122746A1 (en) * | 2001-03-08 | 2002-09-05 | Nihon Kohden Corporation | Sensor for measuring carbon dioxide in respiratory gas |
| US20020133378A1 (en) * | 2000-10-13 | 2002-09-19 | Mault James R. | System and method of integrated calorie management |
| US20020138213A1 (en) * | 2001-03-02 | 2002-09-26 | Mault James R. | System and method of metabolic rate measurement |
| US6468222B1 (en) | 1999-08-02 | 2002-10-22 | Healthetech, Inc. | Metabolic calorimeter employing respiratory gas analysis |
| US6478736B1 (en) | 1999-10-08 | 2002-11-12 | Healthetech, Inc. | Integrated calorie management system |
| US6482158B2 (en) | 2000-05-19 | 2002-11-19 | Healthetech, Inc. | System and method of ultrasonic mammography |
| US6517496B1 (en) | 1999-05-10 | 2003-02-11 | Healthetech, Inc. | Airway-based cardiac output monitor and methods for using same |
| US6552394B2 (en) | 1996-07-09 | 2003-04-22 | Micron Technology, Inc. | Semiconductor transistor devices and structures with halo regions |
| US6572561B2 (en) | 1998-01-16 | 2003-06-03 | Healthetech, Inc. | Respiratory calorimeter |
| US20030126593A1 (en) * | 2002-11-04 | 2003-07-03 | Mault James R. | Interactive physiological monitoring system |
| US6607387B2 (en) | 2000-10-30 | 2003-08-19 | Healthetech, Inc. | Sensor system for diagnosing dental conditions |
| USD478660S1 (en) | 2002-07-01 | 2003-08-19 | Healthetech, Inc. | Disposable mask with sanitation insert for a respiratory analyzer |
| US6612306B1 (en) | 1999-10-13 | 2003-09-02 | Healthetech, Inc. | Respiratory nitric oxide meter |
| US6620106B2 (en) | 2000-09-29 | 2003-09-16 | Healthetech, Inc. | Indirect calorimetry system |
| US6629934B2 (en) | 2000-02-02 | 2003-10-07 | Healthetech, Inc. | Indirect calorimeter for medical applications |
| US6629625B1 (en) * | 2000-11-21 | 2003-10-07 | Coin Acceptors, Inc. | Reflector and anti-fog film in an optical sensor system |
| US6631717B1 (en) * | 1999-10-21 | 2003-10-14 | Ntc Technology Inc. | Re-breathing apparatus for non-invasive cardiac output, method of operation, and ventilator circuit so equipped |
| US20030203991A1 (en) * | 2002-04-30 | 2003-10-30 | Hydromer, Inc. | Coating composition for multiple hydrophilic applications |
| US6648833B2 (en) | 2001-10-15 | 2003-11-18 | David R. Hampton | Respiratory analysis with capnography |
| US20030220579A1 (en) * | 2002-04-01 | 2003-11-27 | Mault James R. | System and method of determining an individualized drug administration protocol |
| US20030226695A1 (en) * | 2000-05-25 | 2003-12-11 | Mault James R. | Weight control method using physical activity based parameters |
| US6790178B1 (en) | 1999-09-24 | 2004-09-14 | Healthetech, Inc. | Physiological monitor and associated computation, display and communication unit |
| US20040182392A1 (en) * | 2003-03-22 | 2004-09-23 | Henning Gerder | Breathing gas tube for a respirator |
| US20040204655A1 (en) * | 2003-04-10 | 2004-10-14 | Burkhard Stock | Breath alcohol measuring device with improved mouthpiece |
| US20040236242A1 (en) * | 2003-05-22 | 2004-11-25 | Graham James E. | Capnograph system with integral controller |
| US20040255943A1 (en) * | 2003-06-23 | 2004-12-23 | Make Morris | System and method for providing a breathing gas |
| US20040260194A1 (en) * | 2003-04-10 | 2004-12-23 | Bayer David J. | Handheld breath tester housing and mouthpiece |
| US20050012053A1 (en) * | 2003-07-16 | 2005-01-20 | O'leary Robert K. | High frequency infrared radiation source |
| US20050109133A1 (en) * | 2003-11-13 | 2005-05-26 | John Lamb | Adaptor means |
| US20050268913A1 (en) * | 2003-06-23 | 2005-12-08 | Make Morris | System and method for providing a breathing gas |
| US20060217625A1 (en) * | 2005-03-25 | 2006-09-28 | Forrester Macquorn R Jr | Mouthpiece for breath tester |
| WO2007051230A1 (en) * | 2005-10-31 | 2007-05-10 | Resmed Ltd | Sensing cuff for breathing apparatus |
| US20070107728A1 (en) * | 2005-11-16 | 2007-05-17 | Cardiopulmonary Technologies, Inc. | Side-stream respiratory gas monitoring system and method |
| US20070132043A1 (en) * | 2002-01-16 | 2007-06-14 | Keith Bradley | Nano-electronic sensors for chemical and biological analytes, including capacitance and bio-membrane devices |
| US20070225612A1 (en) * | 1996-07-15 | 2007-09-27 | Mace Leslie E | Metabolic measurements system including a multiple function airway adapter |
| US20080021339A1 (en) * | 2005-10-27 | 2008-01-24 | Gabriel Jean-Christophe P | Anesthesia monitor, capacitance nanosensors and dynamic sensor sampling method |
| US7335164B2 (en) | 1996-07-15 | 2008-02-26 | Ntc Technology, Inc. | Multiple function airway adapter |
| US20080119753A1 (en) * | 2006-11-16 | 2008-05-22 | Cardiopulmonary Technologies, Inc. | Premature infant side-stream respiratory gas monitoring sensor |
| US7392193B2 (en) | 2000-06-16 | 2008-06-24 | Microlife Corporation | Speech recognition capability for a personal digital assistant |
| US20080221806A1 (en) * | 2005-05-19 | 2008-09-11 | Nanomix, Inc. | Sensor having a thin-film inhibition layer, nitric oxide converter and monitor |
| US20080251079A1 (en) * | 2007-04-13 | 2008-10-16 | Invacare Corporation | Apparatus and method for providing positive airway pressure |
| WO2012033421A1 (en) | 2010-09-10 | 2012-03-15 | Fisher & Paykel Healthcare Limited | A component for conveying gases |
| US8152991B2 (en) | 2005-10-27 | 2012-04-10 | Nanomix, Inc. | Ammonia nanosensors, and environmental control system |
| US8261742B2 (en) | 2007-08-23 | 2012-09-11 | Invacare Corporation | Method and apparatus for adjusting desired pressure in positive airway pressure devices |
| US8993346B2 (en) | 2009-08-07 | 2015-03-31 | Nanomix, Inc. | Magnetic carbon nanotube based biodetection |
| USD727492S1 (en) * | 2012-12-06 | 2015-04-21 | Koninklijke Philips N.V. | Disposable airway adapter for respiratory gas monitoring |
| US20160107121A1 (en) * | 2014-10-17 | 2016-04-21 | Massachusetts Institute Of Technology | Hydrophobic Air-Gap Membrane Distillation |
| AU2017200478B2 (en) * | 2010-09-10 | 2019-01-17 | Fisher & Paykel Healthcare Limited | A component for conveying gases |
| US20200070782A1 (en) * | 2018-09-04 | 2020-03-05 | Ford Global Technologies, Llc | Vehicle rear wiper system |
| US20210146077A1 (en) * | 2018-04-03 | 2021-05-20 | Vincent Medical (Dong Guan) Manufacturing Co., Ltd. | Moisture dissipating cartridge and breathing circuit and breathing system containing such a cartridge |
| AU2022204918B2 (en) * | 2010-09-10 | 2024-10-31 | Fisher & Paykel Healthcare Limited | A component for conveying gases |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5789660A (en) * | 1996-07-15 | 1998-08-04 | Novametrix Medical Systems, Inc. | Multiple function airway adapter |
| US5807636A (en) * | 1994-12-16 | 1998-09-15 | Advanced Surface Technology | Durable hydrophilic surface coatings |
-
1997
- 1997-05-28 US US08/863,637 patent/US6044843A/en not_active Expired - Lifetime
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5807636A (en) * | 1994-12-16 | 1998-09-15 | Advanced Surface Technology | Durable hydrophilic surface coatings |
| US5789660A (en) * | 1996-07-15 | 1998-08-04 | Novametrix Medical Systems, Inc. | Multiple function airway adapter |
Cited By (107)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6552394B2 (en) | 1996-07-09 | 2003-04-22 | Micron Technology, Inc. | Semiconductor transistor devices and structures with halo regions |
| US7335164B2 (en) | 1996-07-15 | 2008-02-26 | Ntc Technology, Inc. | Multiple function airway adapter |
| US20070225612A1 (en) * | 1996-07-15 | 2007-09-27 | Mace Leslie E | Metabolic measurements system including a multiple function airway adapter |
| US6309360B1 (en) | 1997-03-17 | 2001-10-30 | James R. Mault | Respiratory calorimeter |
| US6616615B2 (en) | 1997-03-17 | 2003-09-09 | Healthetech, Inc. | Respiratory calorimeter |
| US6572561B2 (en) | 1998-01-16 | 2003-06-03 | Healthetech, Inc. | Respiratory calorimeter |
| US6645158B2 (en) | 1998-02-05 | 2003-11-11 | Healthetech, Inc. | Metabolic calorimeter employing respiratory gas analysis |
| US6402698B1 (en) | 1998-02-05 | 2002-06-11 | James R. Mault | Metabolic calorimeter employing respiratory gas analysis |
| US6506608B2 (en) | 1998-08-03 | 2003-01-14 | Healthetech, Inc. | Method and apparatus for respiratory gas analysis employing measurement of expired gas mass |
| US6277645B1 (en) | 1998-08-03 | 2001-08-21 | James R. Mault | Method and apparatus for respiratory gas analysis employing measurement of expired gas mass |
| US6258040B1 (en) * | 1998-09-16 | 2001-07-10 | Nihon Kohden Corporation | Airway adapter for non-dispersive infrared gas analyzer |
| US6406435B1 (en) | 1998-11-17 | 2002-06-18 | James R. Mault | Method and apparatus for the non-invasive determination of cardiac output |
| US6190327B1 (en) * | 1999-05-05 | 2001-02-20 | Nonin Medical, Inc. | Disposable airway adapter for use with a carbon dioxide detector |
| US20030167016A1 (en) * | 1999-05-10 | 2003-09-04 | Mault James R. | Airway-based cardiac output monitor and methods for using same |
| US6517496B1 (en) | 1999-05-10 | 2003-02-11 | Healthetech, Inc. | Airway-based cardiac output monitor and methods for using same |
| US6899683B2 (en) | 1999-08-02 | 2005-05-31 | Healthetech, Inc. | Metabolic calorimeter employing respiratory gas analysis |
| US6468222B1 (en) | 1999-08-02 | 2002-10-22 | Healthetech, Inc. | Metabolic calorimeter employing respiratory gas analysis |
| US6955650B2 (en) | 1999-08-02 | 2005-10-18 | Healthetech, Inc. | Metabolic calorimeter employing respiratory gas analysis |
| US6790178B1 (en) | 1999-09-24 | 2004-09-14 | Healthetech, Inc. | Physiological monitor and associated computation, display and communication unit |
| US6478736B1 (en) | 1999-10-08 | 2002-11-12 | Healthetech, Inc. | Integrated calorie management system |
| US6612306B1 (en) | 1999-10-13 | 2003-09-02 | Healthetech, Inc. | Respiratory nitric oxide meter |
| US6631717B1 (en) * | 1999-10-21 | 2003-10-14 | Ntc Technology Inc. | Re-breathing apparatus for non-invasive cardiac output, method of operation, and ventilator circuit so equipped |
| US6534769B1 (en) | 1999-12-31 | 2003-03-18 | Ge Medical Systems Information Technologies, Inc. | Low cost main stream gas analyzer system |
| EP1112716A3 (en) * | 1999-12-31 | 2001-11-07 | GE Marquette Medical Systems, Inc. | Low cost main stream gas analyzer system |
| US6629934B2 (en) | 2000-02-02 | 2003-10-07 | Healthetech, Inc. | Indirect calorimeter for medical applications |
| US6482158B2 (en) | 2000-05-19 | 2002-11-19 | Healthetech, Inc. | System and method of ultrasonic mammography |
| US20030226695A1 (en) * | 2000-05-25 | 2003-12-11 | Mault James R. | Weight control method using physical activity based parameters |
| US7392193B2 (en) | 2000-06-16 | 2008-06-24 | Microlife Corporation | Speech recognition capability for a personal digital assistant |
| US20020047867A1 (en) * | 2000-09-07 | 2002-04-25 | Mault James R | Image based diet logging |
| US6990980B2 (en) | 2000-09-28 | 2006-01-31 | Invacare Corporation | Carbon dioxide-based Bi-level CPAP control |
| US8640701B2 (en) | 2000-09-28 | 2014-02-04 | Invacare Corporation | Carbon dioxide-based bi-level CPAP control |
| US20020104536A1 (en) * | 2000-09-28 | 2002-08-08 | Richey Joseph B. | Carbon dioxide-based Bi-level CPAP Control |
| US20050279358A1 (en) * | 2000-09-28 | 2005-12-22 | Richey Joseph B Ii | Carbon dioxide-based bi-level CPAP control |
| US6620106B2 (en) | 2000-09-29 | 2003-09-16 | Healthetech, Inc. | Indirect calorimetry system |
| US20020133378A1 (en) * | 2000-10-13 | 2002-09-19 | Mault James R. | System and method of integrated calorie management |
| US6607387B2 (en) | 2000-10-30 | 2003-08-19 | Healthetech, Inc. | Sensor system for diagnosing dental conditions |
| US20020055857A1 (en) * | 2000-10-31 | 2002-05-09 | Mault James R. | Method of assisting individuals in lifestyle control programs conducive to good health |
| US6629625B1 (en) * | 2000-11-21 | 2003-10-07 | Coin Acceptors, Inc. | Reflector and anti-fog film in an optical sensor system |
| US20020138213A1 (en) * | 2001-03-02 | 2002-09-26 | Mault James R. | System and method of metabolic rate measurement |
| US8636956B2 (en) | 2001-03-08 | 2014-01-28 | Nihon Kohden Corporation | Sensor for measuring carbon dioxide in respiratory gas |
| US7462154B2 (en) * | 2001-03-08 | 2008-12-09 | Nihon Kohden Corporation | Sensor for measuring carbon dioxide in respiratory gas |
| US20090088657A1 (en) * | 2001-03-08 | 2009-04-02 | Nihon Kohden Corporation | Sensor for measuring carbon dioxide in respiratory gas |
| US20020122746A1 (en) * | 2001-03-08 | 2002-09-05 | Nihon Kohden Corporation | Sensor for measuring carbon dioxide in respiratory gas |
| US6648833B2 (en) | 2001-10-15 | 2003-11-18 | David R. Hampton | Respiratory analysis with capnography |
| US8154093B2 (en) | 2002-01-16 | 2012-04-10 | Nanomix, Inc. | Nano-electronic sensors for chemical and biological analytes, including capacitance and bio-membrane devices |
| US9103775B2 (en) | 2002-01-16 | 2015-08-11 | Nanomix, Inc. | Nano-electronic sensors for chemical and biological analytes, including capacitance and bio-membrane devices |
| US20070132043A1 (en) * | 2002-01-16 | 2007-06-14 | Keith Bradley | Nano-electronic sensors for chemical and biological analytes, including capacitance and bio-membrane devices |
| US20030220579A1 (en) * | 2002-04-01 | 2003-11-27 | Mault James R. | System and method of determining an individualized drug administration protocol |
| US7291114B2 (en) | 2002-04-01 | 2007-11-06 | Microlife Corporation | System and method of determining an individualized drug administration protocol |
| US7008979B2 (en) | 2002-04-30 | 2006-03-07 | Hydromer, Inc. | Coating composition for multiple hydrophilic applications |
| US20030203991A1 (en) * | 2002-04-30 | 2003-10-30 | Hydromer, Inc. | Coating composition for multiple hydrophilic applications |
| US9291613B2 (en) | 2002-06-21 | 2016-03-22 | Nanomix, Inc. | Sensor having a thin-film inhibition layer |
| USD478660S1 (en) | 2002-07-01 | 2003-08-19 | Healthetech, Inc. | Disposable mask with sanitation insert for a respiratory analyzer |
| US20100085067A1 (en) * | 2002-09-05 | 2010-04-08 | Nanomix, Inc. | Anesthesia monitor, capacitance nanosensors and dynamic sensor sampling method |
| US20030126593A1 (en) * | 2002-11-04 | 2003-07-03 | Mault James R. | Interactive physiological monitoring system |
| US20040182392A1 (en) * | 2003-03-22 | 2004-09-23 | Henning Gerder | Breathing gas tube for a respirator |
| US7647926B2 (en) * | 2003-03-22 | 2010-01-19 | Drägerwerk AG | Breathing gas tube for a respirator |
| US20040204655A1 (en) * | 2003-04-10 | 2004-10-14 | Burkhard Stock | Breath alcohol measuring device with improved mouthpiece |
| US7749169B2 (en) * | 2003-04-10 | 2010-07-06 | Intoximeters, Inc. | Handheld breath tester housing and mouthpiece |
| US7993281B2 (en) * | 2003-04-10 | 2011-08-09 | Dräger Safety AG & Co. KGaA | Breath alcohol measuring device with improved mouthpiece |
| US20060206034A1 (en) * | 2003-04-10 | 2006-09-14 | Burkhard Stock | Breath alcohol measuring device with improved mouthpiece |
| AU2004201163B2 (en) * | 2003-04-10 | 2009-07-16 | Draeger Safety Ag & Co Kgaa | Breath alcohol measuring device with improved mouthpiece |
| US20040260194A1 (en) * | 2003-04-10 | 2004-12-23 | Bayer David J. | Handheld breath tester housing and mouthpiece |
| US20040236242A1 (en) * | 2003-05-22 | 2004-11-25 | Graham James E. | Capnograph system with integral controller |
| US7152598B2 (en) | 2003-06-23 | 2006-12-26 | Invacare Corporation | System and method for providing a breathing gas |
| US8066004B2 (en) | 2003-06-23 | 2011-11-29 | Invacare Corporation | System and method for providing a breathing gas |
| US20040255943A1 (en) * | 2003-06-23 | 2004-12-23 | Make Morris | System and method for providing a breathing gas |
| US7621270B2 (en) | 2003-06-23 | 2009-11-24 | Invacare Corp. | System and method for providing a breathing gas |
| US20100065055A1 (en) * | 2003-06-23 | 2010-03-18 | Invacare Corporation | System and method for providing a breathing gas |
| US20050268913A1 (en) * | 2003-06-23 | 2005-12-08 | Make Morris | System and method for providing a breathing gas |
| US20050012053A1 (en) * | 2003-07-16 | 2005-01-20 | O'leary Robert K. | High frequency infrared radiation source |
| US6878938B2 (en) | 2003-07-16 | 2005-04-12 | Perkinelmer, Inc. | High frequency infrared radiation source |
| US20050109133A1 (en) * | 2003-11-13 | 2005-05-26 | John Lamb | Adaptor means |
| US20060217625A1 (en) * | 2005-03-25 | 2006-09-28 | Forrester Macquorn R Jr | Mouthpiece for breath tester |
| US8754454B2 (en) | 2005-05-19 | 2014-06-17 | Nanomix, Inc. | Sensor having a thin-film inhibition layer |
| US20080221806A1 (en) * | 2005-05-19 | 2008-09-11 | Nanomix, Inc. | Sensor having a thin-film inhibition layer, nitric oxide converter and monitor |
| US7948041B2 (en) | 2005-05-19 | 2011-05-24 | Nanomix, Inc. | Sensor having a thin-film inhibition layer |
| US8152991B2 (en) | 2005-10-27 | 2012-04-10 | Nanomix, Inc. | Ammonia nanosensors, and environmental control system |
| US20080021339A1 (en) * | 2005-10-27 | 2008-01-24 | Gabriel Jean-Christophe P | Anesthesia monitor, capacitance nanosensors and dynamic sensor sampling method |
| WO2007051230A1 (en) * | 2005-10-31 | 2007-05-10 | Resmed Ltd | Sensing cuff for breathing apparatus |
| US20070107728A1 (en) * | 2005-11-16 | 2007-05-17 | Cardiopulmonary Technologies, Inc. | Side-stream respiratory gas monitoring system and method |
| US8459261B2 (en) | 2005-11-16 | 2013-06-11 | Treymed, Inc. | Side-stream respiratory gas monitoring system and method |
| US20080119753A1 (en) * | 2006-11-16 | 2008-05-22 | Cardiopulmonary Technologies, Inc. | Premature infant side-stream respiratory gas monitoring sensor |
| US20080251079A1 (en) * | 2007-04-13 | 2008-10-16 | Invacare Corporation | Apparatus and method for providing positive airway pressure |
| US8261742B2 (en) | 2007-08-23 | 2012-09-11 | Invacare Corporation | Method and apparatus for adjusting desired pressure in positive airway pressure devices |
| US8993346B2 (en) | 2009-08-07 | 2015-03-31 | Nanomix, Inc. | Magnetic carbon nanotube based biodetection |
| GB2503357B (en) * | 2010-09-10 | 2014-08-27 | Fisher & Paykel Healthcare Ltd | A component for conveying gases |
| AU2017200478B2 (en) * | 2010-09-10 | 2019-01-17 | Fisher & Paykel Healthcare Limited | A component for conveying gases |
| GB2496569B (en) * | 2010-09-10 | 2014-08-27 | Fisher & Paykel Healthcare Ltd | A component for conveying gases |
| JP2013541968A (en) * | 2010-09-10 | 2013-11-21 | フィッシャー アンド ペイケル ヘルスケア リミテッド | Components for transporting gas |
| CN103180004A (en) * | 2010-09-10 | 2013-06-26 | 费雪派克医疗保健有限公司 | Components for conveying gases |
| AU2022204918B2 (en) * | 2010-09-10 | 2024-10-31 | Fisher & Paykel Healthcare Limited | A component for conveying gases |
| GB2496569A (en) * | 2010-09-10 | 2013-05-15 | Fisher & Paykel Healthcare Ltd | A component for conveying gases |
| WO2012033421A1 (en) | 2010-09-10 | 2012-03-15 | Fisher & Paykel Healthcare Limited | A component for conveying gases |
| EP4223344A1 (en) * | 2010-09-10 | 2023-08-09 | Fisher & Paykel Healthcare Limited | A component for conveying gases |
| EP2613836A4 (en) * | 2010-09-10 | 2017-08-02 | Fisher & Paykel Healthcare Limited | A component for conveying gases |
| US11358318B2 (en) | 2010-09-10 | 2022-06-14 | Fisher & Paykel Healthcare Limited | Component for conveying gases |
| GB2503357A (en) * | 2010-09-10 | 2013-12-25 | Fisher & Paykel Healthcare Ltd | Foamed wall breathing tube |
| US10449318B2 (en) | 2010-09-10 | 2019-10-22 | Fisher & Paykel Healthcare Limited | Component for conveying gases |
| AU2018278847B2 (en) * | 2010-09-10 | 2020-07-02 | Fisher & Paykel Healthcare Limited | A component for conveying gases |
| USD727492S1 (en) * | 2012-12-06 | 2015-04-21 | Koninklijke Philips N.V. | Disposable airway adapter for respiratory gas monitoring |
| US9751047B2 (en) * | 2014-10-17 | 2017-09-05 | Massachusetts Institute Of Technology | Hydrophobic air-gap membrane distillation |
| US20160107121A1 (en) * | 2014-10-17 | 2016-04-21 | Massachusetts Institute Of Technology | Hydrophobic Air-Gap Membrane Distillation |
| US20210146077A1 (en) * | 2018-04-03 | 2021-05-20 | Vincent Medical (Dong Guan) Manufacturing Co., Ltd. | Moisture dissipating cartridge and breathing circuit and breathing system containing such a cartridge |
| US12036364B2 (en) * | 2018-04-03 | 2024-07-16 | Vincent Medical (Dong Guan) Manufacturing Co., Ltd | Moisture dissipating cartridge and breathing circuit and breathing system containing such a cartridge |
| US20200070782A1 (en) * | 2018-09-04 | 2020-03-05 | Ford Global Technologies, Llc | Vehicle rear wiper system |
| US10793115B2 (en) * | 2018-09-04 | 2020-10-06 | Ford Global Technologies, Llc | Vehicle rear wiper system |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6044843A (en) | Moisture resistant airway adapter for monitoring constituent gases | |
| US7335164B2 (en) | Multiple function airway adapter | |
| RU2476148C2 (en) | Methabolic measuring system with multifunctional adapter for respiratory tract | |
| US4958075A (en) | Gas analyzer | |
| US6419634B1 (en) | Condensate colorimetric nitrogen oxide analyzer | |
| EP2395915B1 (en) | Breath analysis | |
| US4509359A (en) | Method and apparatus for measuring the concentration of a given component in a gas inhaled and/or exhaled by a patient | |
| US6190327B1 (en) | Disposable airway adapter for use with a carbon dioxide detector | |
| US9999373B2 (en) | On-airway pulmonary function tester | |
| US20100036272A1 (en) | Metabolic measure system including a multiple function airway adapter | |
| US20080027344A1 (en) | Modular sidestream gas sampling assembly | |
| JP4469491B2 (en) | Low cost mainstream gas analyzer system | |
| US20230266244A1 (en) | Ndir sensor, sampling method and system for breath analysis | |
| US7556039B1 (en) | Sidestream gas sampling system using a capillary tube flow sensor | |
| CN110522451B (en) | Method and system for measuring dispersion amount of CO in multi-component gas | |
| EP1420692B1 (en) | Device for quantitative analysis of respiratory gases, comprising a passive respiratory gas humidifyer, where rays of light are transmitted through a dehumified gas flow | |
| EP1420691B1 (en) | Device for quantitative analysis of respiratory gases | |
| US20040215096A1 (en) | Device at quantitative analysis of respiratory gases | |
| CA2099740A1 (en) | Expired gas sampling method and expired gas collecting tube | |
| WO1989003523A1 (en) | Gas analyzer | |
| Burte et al. | Microsystems for measurement and dosage of volatile anesthetics and respirative gases in anesthetic equipment |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: NELLCOR PURITAN BENNETT INCORPORATED, CALIFORNIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:O'NEAL, MICHAEL P.;FLEWELLING, ROSS;CULVER-BARRETT, KRISTINE J., EXECUTOR OF THE ESTATE OF CULVER, JOHN A., DECEASED;REEL/FRAME:008588/0584;SIGNING DATES FROM 19970415 TO 19970501 |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| FEPP | Fee payment procedure |
Free format text: PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| FPAY | Fee payment |
Year of fee payment: 8 |
|
| FPAY | Fee payment |
Year of fee payment: 12 |