US20060235282A1 - Methods for the treatment of mammals with abnormal glucose tolerance - Google Patents
Methods for the treatment of mammals with abnormal glucose tolerance Download PDFInfo
- Publication number
- US20060235282A1 US20060235282A1 US11/377,179 US37717906A US2006235282A1 US 20060235282 A1 US20060235282 A1 US 20060235282A1 US 37717906 A US37717906 A US 37717906A US 2006235282 A1 US2006235282 A1 US 2006235282A1
- Authority
- US
- United States
- Prior art keywords
- evaluation
- patient
- diet
- glucose
- diameter
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000000034 method Methods 0.000 title claims abstract description 47
- 206010018429 Glucose tolerance impaired Diseases 0.000 title claims abstract description 13
- 241000124008 Mammalia Species 0.000 title abstract description 3
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 claims abstract description 42
- 206010022489 Insulin Resistance Diseases 0.000 claims abstract description 30
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims abstract description 22
- 102000004877 Insulin Human genes 0.000 claims abstract description 21
- 108090001061 Insulin Proteins 0.000 claims abstract description 21
- 229940125396 insulin Drugs 0.000 claims abstract description 21
- 208000002705 Glucose Intolerance Diseases 0.000 claims abstract description 13
- 206010056997 Impaired fasting glucose Diseases 0.000 claims abstract description 13
- 206010012601 diabetes mellitus Diseases 0.000 claims abstract description 13
- 201000009104 prediabetes syndrome Diseases 0.000 claims abstract description 13
- 206010020772 Hypertension Diseases 0.000 claims abstract description 11
- 230000004060 metabolic process Effects 0.000 claims abstract description 5
- 208000001145 Metabolic Syndrome Diseases 0.000 claims abstract description 4
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 claims abstract description 4
- 208000021959 Abnormal metabolism Diseases 0.000 claims abstract description 3
- 238000011156 evaluation Methods 0.000 claims description 42
- 235000005911 diet Nutrition 0.000 claims description 33
- 230000037213 diet Effects 0.000 claims description 33
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 30
- 239000008103 glucose Substances 0.000 claims description 30
- 235000013305 food Nutrition 0.000 claims description 25
- 230000037081 physical activity Effects 0.000 claims description 16
- 238000000554 physical therapy Methods 0.000 claims description 14
- 239000008280 blood Substances 0.000 claims description 13
- 210000004369 blood Anatomy 0.000 claims description 13
- 235000016709 nutrition Nutrition 0.000 claims description 12
- 238000005259 measurement Methods 0.000 claims description 9
- 230000000694 effects Effects 0.000 claims description 7
- 210000003205 muscle Anatomy 0.000 claims description 6
- 235000000346 sugar Nutrition 0.000 claims description 6
- 230000036772 blood pressure Effects 0.000 claims description 5
- 208000029078 coronary artery disease Diseases 0.000 claims description 5
- 208000035150 Hypercholesterolemia Diseases 0.000 claims description 4
- 229940079593 drug Drugs 0.000 claims description 4
- 239000003814 drug Substances 0.000 claims description 4
- 230000033001 locomotion Effects 0.000 claims description 4
- 239000000203 mixture Substances 0.000 claims description 4
- 238000012360 testing method Methods 0.000 claims description 4
- 230000002159 abnormal effect Effects 0.000 claims description 3
- 150000001720 carbohydrates Chemical class 0.000 claims description 3
- 235000014633 carbohydrates Nutrition 0.000 claims description 3
- 230000002802 cardiorespiratory effect Effects 0.000 claims description 3
- 230000012010 growth Effects 0.000 claims description 3
- 230000000366 juvenile effect Effects 0.000 claims description 3
- 238000012544 monitoring process Methods 0.000 claims description 3
- 230000008450 motivation Effects 0.000 claims description 3
- 230000000391 smoking effect Effects 0.000 claims description 3
- 208000007848 Alcoholism Diseases 0.000 claims description 2
- 208000000412 Avitaminosis Diseases 0.000 claims description 2
- 206010013654 Drug abuse Diseases 0.000 claims description 2
- 206010013710 Drug interaction Diseases 0.000 claims description 2
- 208000030814 Eating disease Diseases 0.000 claims description 2
- 208000019454 Feeding and Eating disease Diseases 0.000 claims description 2
- 206010016948 Food interaction Diseases 0.000 claims description 2
- 206010061291 Mineral deficiency Diseases 0.000 claims description 2
- 206010047627 Vitamin deficiencies Diseases 0.000 claims description 2
- 201000007930 alcohol dependence Diseases 0.000 claims description 2
- 210000001124 body fluid Anatomy 0.000 claims description 2
- 239000010839 body fluid Substances 0.000 claims description 2
- 235000021152 breakfast Nutrition 0.000 claims description 2
- 235000014632 disordered eating Nutrition 0.000 claims description 2
- 235000006694 eating habits Nutrition 0.000 claims description 2
- 208000004104 gestational diabetes Diseases 0.000 claims description 2
- 238000002483 medication Methods 0.000 claims description 2
- 208000011117 substance-related disease Diseases 0.000 claims description 2
- 230000000007 visual effect Effects 0.000 claims description 2
- 229930003231 vitamin Natural products 0.000 claims description 2
- 235000013343 vitamin Nutrition 0.000 claims description 2
- 239000011782 vitamin Substances 0.000 claims description 2
- 229940088594 vitamin Drugs 0.000 claims description 2
- 150000003722 vitamin derivatives Chemical class 0.000 claims description 2
- 244000309466 calf Species 0.000 claims 2
- 210000000245 forearm Anatomy 0.000 claims 2
- 210000003127 knee Anatomy 0.000 claims 2
- 230000000284 resting effect Effects 0.000 claims 2
- 210000000689 upper leg Anatomy 0.000 claims 2
- 210000001015 abdomen Anatomy 0.000 claims 1
- 210000003195 fascia Anatomy 0.000 claims 1
- 210000002414 leg Anatomy 0.000 claims 1
- 230000004153 glucose metabolism Effects 0.000 abstract description 2
- 208000008589 Obesity Diseases 0.000 description 16
- 235000020824 obesity Nutrition 0.000 description 16
- 206010033307 Overweight Diseases 0.000 description 13
- 230000007423 decrease Effects 0.000 description 8
- 229920002472 Starch Polymers 0.000 description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 7
- 239000003925 fat Substances 0.000 description 7
- 235000019698 starch Nutrition 0.000 description 7
- 241000251468 Actinopterygii Species 0.000 description 6
- 235000019197 fats Nutrition 0.000 description 6
- 235000019688 fish Nutrition 0.000 description 6
- 235000004426 flaxseed Nutrition 0.000 description 6
- 230000002641 glycemic effect Effects 0.000 description 6
- 230000036541 health Effects 0.000 description 6
- 230000003247 decreasing effect Effects 0.000 description 5
- 238000011161 development Methods 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- 230000002526 effect on cardiovascular system Effects 0.000 description 5
- 230000001610 euglycemic effect Effects 0.000 description 5
- 239000000835 fiber Substances 0.000 description 5
- 235000012054 meals Nutrition 0.000 description 5
- 235000013311 vegetables Nutrition 0.000 description 5
- 208000017667 Chronic Disease Diseases 0.000 description 4
- 210000000577 adipose tissue Anatomy 0.000 description 4
- 235000019577 caloric intake Nutrition 0.000 description 4
- 235000013339 cereals Nutrition 0.000 description 4
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 4
- 235000020660 omega-3 fatty acid Nutrition 0.000 description 4
- 229940012843 omega-3 fatty acid Drugs 0.000 description 4
- 239000006014 omega-3 oil Substances 0.000 description 4
- 235000018102 proteins Nutrition 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 238000012552 review Methods 0.000 description 4
- 235000021309 simple sugar Nutrition 0.000 description 4
- 230000004580 weight loss Effects 0.000 description 4
- 241000196324 Embryophyta Species 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 229930091371 Fructose Natural products 0.000 description 3
- 239000005715 Fructose Substances 0.000 description 3
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- 230000001154 acute effect Effects 0.000 description 3
- 235000021120 animal protein Nutrition 0.000 description 3
- 235000013399 edible fruits Nutrition 0.000 description 3
- 235000021323 fish oil Nutrition 0.000 description 3
- 229940013317 fish oils Drugs 0.000 description 3
- 238000012423 maintenance Methods 0.000 description 3
- 230000002503 metabolic effect Effects 0.000 description 3
- 230000035764 nutrition Effects 0.000 description 3
- 229940038580 oat bran Drugs 0.000 description 3
- 238000007410 oral glucose tolerance test Methods 0.000 description 3
- 230000003449 preventive effect Effects 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 230000009469 supplementation Effects 0.000 description 3
- 235000021076 total caloric intake Nutrition 0.000 description 3
- 235000020985 whole grains Nutrition 0.000 description 3
- MJYQFWSXKFLTAY-OVEQLNGDSA-N (2r,3r)-2,3-bis[(4-hydroxy-3-methoxyphenyl)methyl]butane-1,4-diol;(2r,3r,4s,5s,6r)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O.C1=C(O)C(OC)=CC(C[C@@H](CO)[C@H](CO)CC=2C=C(OC)C(O)=CC=2)=C1 MJYQFWSXKFLTAY-OVEQLNGDSA-N 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Natural products OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- 206010061818 Disease progression Diseases 0.000 description 2
- 208000007683 Pediatric Obesity Diseases 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 230000005750 disease progression Effects 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 235000021588 free fatty acids Nutrition 0.000 description 2
- 238000007446 glucose tolerance test Methods 0.000 description 2
- 230000005802 health problem Effects 0.000 description 2
- 230000002440 hepatic effect Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 235000021003 saturated fats Nutrition 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 230000007704 transition Effects 0.000 description 2
- 150000003626 triacylglycerols Chemical class 0.000 description 2
- FPIPGXGPPPQFEQ-UHFFFAOYSA-N 13-cis retinol Natural products OCC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-UHFFFAOYSA-N 0.000 description 1
- 208000006096 Attention Deficit Disorder with Hyperactivity Diseases 0.000 description 1
- 208000036864 Attention deficit/hyperactivity disease Diseases 0.000 description 1
- 241000972773 Aulopiformes Species 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 208000032841 Bulimia Diseases 0.000 description 1
- 206010006550 Bulimia nervosa Diseases 0.000 description 1
- 101100468275 Caenorhabditis elegans rep-1 gene Proteins 0.000 description 1
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 description 1
- 208000032928 Dyslipidaemia Diseases 0.000 description 1
- 206010014476 Elevated cholesterol Diseases 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 201000003741 Gastrointestinal carcinoma Diseases 0.000 description 1
- 108010010234 HDL Lipoproteins Proteins 0.000 description 1
- 108010021075 HDL2 Lipoproteins Proteins 0.000 description 1
- 208000017170 Lipid metabolism disease Diseases 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 208000002720 Malnutrition Diseases 0.000 description 1
- 201000002451 Overnutrition Diseases 0.000 description 1
- 208000017442 Retinal disease Diseases 0.000 description 1
- 206010038923 Retinopathy Diseases 0.000 description 1
- 241000220317 Rosa Species 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- JLRGJRBPOGGCBT-UHFFFAOYSA-N Tolbutamide Chemical compound CCCCNC(=O)NS(=O)(=O)C1=CC=C(C)C=C1 JLRGJRBPOGGCBT-UHFFFAOYSA-N 0.000 description 1
- FPIPGXGPPPQFEQ-BOOMUCAASA-N Vitamin A Natural products OC/C=C(/C)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-BOOMUCAASA-N 0.000 description 1
- 229930003268 Vitamin C Natural products 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- 229930003448 Vitamin K Natural products 0.000 description 1
- 230000003187 abdominal effect Effects 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 1
- 208000022531 anorexia Diseases 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 230000005806 chronic musculo-skeletal problem Effects 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 235000009508 confectionery Nutrition 0.000 description 1
- 235000013365 dairy product Nutrition 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 206010061428 decreased appetite Diseases 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 235000013325 dietary fiber Nutrition 0.000 description 1
- 235000021004 dietary regimen Nutrition 0.000 description 1
- 235000005686 eating Nutrition 0.000 description 1
- 230000002124 endocrine Effects 0.000 description 1
- 210000004696 endometrium Anatomy 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 235000013410 fast food Nutrition 0.000 description 1
- 235000013861 fat-free Nutrition 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 230000020764 fibrinolysis Effects 0.000 description 1
- 235000015219 food category Nutrition 0.000 description 1
- 210000000232 gallbladder Anatomy 0.000 description 1
- 208000020694 gallbladder disease Diseases 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000004110 gluconeogenesis Effects 0.000 description 1
- 230000009229 glucose formation Effects 0.000 description 1
- 230000014101 glucose homeostasis Effects 0.000 description 1
- 230000004190 glucose uptake Effects 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 230000000910 hyperinsulinemic effect Effects 0.000 description 1
- 201000008980 hyperinsulinism Diseases 0.000 description 1
- 208000000509 infertility Diseases 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 231100000535 infertility Toxicity 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000006362 insulin response pathway Effects 0.000 description 1
- 201000002313 intestinal cancer Diseases 0.000 description 1
- CJWQYWQDLBZGPD-UHFFFAOYSA-N isoflavone Natural products C1=C(OC)C(OC)=CC(OC)=C1C1=COC2=C(C=CC(C)(C)O3)C3=C(OC)C=C2C1=O CJWQYWQDLBZGPD-UHFFFAOYSA-N 0.000 description 1
- 150000002515 isoflavone derivatives Chemical class 0.000 description 1
- 235000008696 isoflavones Nutrition 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 208000017169 kidney disease Diseases 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 230000004130 lipolysis Effects 0.000 description 1
- 238000013178 mathematical model Methods 0.000 description 1
- 235000013372 meat Nutrition 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 235000021281 monounsaturated fatty acids Nutrition 0.000 description 1
- 208000031225 myocardial ischemia Diseases 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- 235000020823 overnutrition Nutrition 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- SHUZOJHMOBOZST-UHFFFAOYSA-N phylloquinone Natural products CC(C)CCCCC(C)CCC(C)CCCC(=CCC1=C(C)C(=O)c2ccccc2C1=O)C SHUZOJHMOBOZST-UHFFFAOYSA-N 0.000 description 1
- 235000020777 polyunsaturated fatty acids Nutrition 0.000 description 1
- 230000000291 postprandial effect Effects 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000009023 proprioceptive sensation Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000005801 respiratory difficulty Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 235000019515 salmon Nutrition 0.000 description 1
- 235000003441 saturated fatty acids Nutrition 0.000 description 1
- 150000004671 saturated fatty acids Chemical class 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 230000005808 skin problem Effects 0.000 description 1
- 201000002859 sleep apnea Diseases 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 229960005371 tolbutamide Drugs 0.000 description 1
- 238000012549 training Methods 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 235000000112 undernutrition Nutrition 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 235000021126 varied diet Nutrition 0.000 description 1
- 235000019155 vitamin A Nutrition 0.000 description 1
- 239000011719 vitamin A Substances 0.000 description 1
- 235000019154 vitamin C Nutrition 0.000 description 1
- 239000011718 vitamin C Substances 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 235000019168 vitamin K Nutrition 0.000 description 1
- 239000011712 vitamin K Substances 0.000 description 1
- 150000003721 vitamin K derivatives Chemical class 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H20/00—ICT specially adapted for therapies or health-improving plans, e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance
- G16H20/30—ICT specially adapted for therapies or health-improving plans, e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance relating to physical therapies or activities, e.g. physiotherapy, acupressure or exercising
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H20/00—ICT specially adapted for therapies or health-improving plans, e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance
- G16H20/60—ICT specially adapted for therapies or health-improving plans, e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance relating to nutrition control, e.g. diets
Definitions
- the present invention relates to methods for improving insulin sensitivity and/or glucose metabolism in individuals with abnormal glucose tolerance. Such methods may be used in treating conditions associated with abnormal glucose tolerance and metabolism in mammals, including insulin resistance, insulin resistant metabolic syndrome, impaired glucose tolerance, impaired fasting glucose, hypertension, hypercholesterolemia and diabetes, particularly Type 2 diabetes.
- BMI body mass index
- Raised BMI is also thought to increase the risks of cancer of the breast, colon, prostrate, endometrium, kidney and gallbladder.
- Chronic overweight and obesity contribute significantly to osteoarthritis, a major cause of disability in adults.
- obesity should be considered a disease in its own right, it is also one of the key risk factors for other chronic diseases together with smoking, high blood pressure and high blood cholesterol.
- BMI levels of 22-23 kg/m 2 are found in Africa and Asia, while levels of 25-27 kg/m 2 are prevalent across North America, Europe, and in some Latin American, North African and Pacific Island countries. BMI increases amongst middle-aged elderly people, who are at the greatest risk of health complications. In countries undergoing nutrition transition, over nutrition often co-exists with undernutrition.
- BMI The distribution of BMI is shifting upwards in many populations. And recent studies have shown that people who were undernourished in early life and then become obese in adulthood, tend to develop conditions such as high blood pressure, heart disease and diabetes at an earlier age and in more severe form than those who were never undernourished.
- Childhood obesity is already epidemic in some areas and on the rise in others. An estimated 22 million children under five are estimated to be overweight worldwide. According to the US Surgeon General, in the USA the number of overweight children has doubled and the number of overweight adolescents has trebled since 1980. The prevalence of obese children aged 6-11 years has more than doubled since the 1960s. Obesity prevalence in juveniles aged 12-17 has increased dramatically from 5% to 13% in boys and from 5% to 9% in girls between 1966-70 and 1988-91 in the USA. The problem is global and increasingly extends into the developing world; for example, in Thailand the prevalence of obesity in 5-to-12 year olds children rose froml2.2% to 15-6% in just two years.
- Obesity accounts for 2-6% of total health care costs in several developed countries; some estimates put the figure as high as 7%. The true costs are undoubtedly much greater as not all obesity-related conditions are included in the calculations.
- Type 2 diabetes and hypertension rises steeply with increasing body fatness. Confined to older adults for most of the 20th century, this disease now affects obese children even before puberty. Approximately 85% of people with diabetes are type 2, and of these, 90% are obese or overweight. And this is increasingly becoming a developing world problem. In 1995, the Emerging Market economiess had the highest number of diabetics. If current trends continue, India and the Middle Eastern crescent will likely have taken over by 2025. Large increases would likely also be observed in China, Latin America and the Caribbean, and the rest of Asia.
- Abnormal glucose tolerance refers to metabolic stages intermediary to normal glucose homeostasis and Type 2 diabetes; this includes conditions like impaired glucose tolerance (IGT) and impaired fasting glucose (JFG), where glucose values are above the conventional normal range and are often accompanied by a decrease in insulin sensitivity.
- Impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) are transient, intermediate stages in the development of Type 2 diabetes.
- IGT impaired glucose tolerance
- IGF impaired fasting glucose
- IGT impaired fasting glucose
- Abnormal glucose tolerance and decreased insulin sensitivity can be attributed to a wide range of causes including obesity, age, physical activity level, certain medication or drugs, genetic factors, and some endocrine related disorders.
- the truncal distribution of weight as determined by a high waist to hip ratio (WHR) is a good predictor of abnormal insulin sensitivity, and there is an excellent correlation between a high body mass index (BMI) and decreased insulin sensitivity.
- WHR waist to hip ratio
- BMI body mass index
- Approximately 33% of the population in the United States is obese, and the majority of these individuals have decreased insulin sensitivity, are hyperinsulinemic, and often have abnormal glucose tolerance.
- Impaired fasting glucose is defined by the American Diabetes Association as a fasting blood glucose in the range of 110 mg/dL to 125 mg/dL. IFG is determined by analysis of a plasma sample for glucose after a 10-16 hour fast. This is an easy and quick way to determine if there is a problem with glucose tolerance and metabolism.
- Impaired glucose tolerance is determined by the administration of a standard oral glucose tolerance test (OGT) (World Health Org., Diabetes Mellitus, Tech. Rep. Ser., no. 727 (1985)).
- OGT oral glucose tolerance test
- a measured amount of glucose 75 grams
- blood glucose levels are measured every 30 minutes, usually for 2 or 3 hours.
- the blood glucose values will rise during the first part of the test and then rapidly return to basal levels.
- the post prandial glucose levels will rise above the normal range, producing a 2-hour glucose value of 140-199 mg/dL, and return to basal levels at a slow rate.
- Abnormal glucose tolerance is caused in part by inadequate utilization of glucose in the periphery—at the site of the muscles.
- high fasting glucose values seen with impaired fasting glucose, suggest that hepatic glucose production is not being effectively regulated.
- the underlying cause of this abnormal glucose tolerance is characterized by a decrease in insulin sensitivity.
- Insulin sensitivity is a measurement of insulin's ability to produce a biological response; specifically, in the case of glucose regulation, insulin sensitivity is a measurement of insulin's ability to promote the clearance and utilization of glucose.
- a decrease in insulin sensitivity will result in a prolonged elevation of glucose levels and the release of additional insulin to try and achieve a euglycemic state.
- This compensatory hyperinsulinemia will effect insulin's ability to suppress lipolysis in adipose tissue, thus causing an increase in free fatty acids and ultimately resulting in the disruption of normal lipid profiles which could lead to coronary artery disease.
- the increase in free fatty acids will also inhibit insulin-stimulated glucose utilization in the muscle and stimulate hepatic gluconeogenesis. This leads to increased blood glucose and will eventually result in the development of impaired glucose tolerance or impaired fasting glucose and ultimately, if unchecked, the development of Type 2 diabetes. Improving insulin sensitivity will restore overall glucose control and decrease the risk of cardiovascular disease.
- the level of insulin sensitivity can be measured to varying degrees by three methods: fasting plasma insulin, the euglycemic clamp, and the frequently sampled intravenous glucose tolerance test (FSIGTT). With the use of any of these techniques there is a wide range of insulin sensitivity, with overlapping values characterizing individuals with normal, abnormal glucose tolerance and Type 2 diabetes.
- FSIGTT frequently sampled intravenous glucose tolerance test
- One method of determining insulin sensitivity is by fasting plasma insulin values. Simply, a high fasting insulin value correlates with decreased insulin sensitivity.
- the euglycemic clamp test procedure involves infusing glucose at a variable rate in order to obtain a constant plasma glucose concentration. This rate of glucose infusion is equal to the overall rate of basal glucose disposal in the body. When insulin is also infused, the glucose infusion rate reflects insulin mediated glucose uptake. This is a precise and reproducible way to determine insulin sensitivity, albeit the euglycemic clamp method is time-consuming and complicated to perform.
- a third method of determining insulin sensitivity involves frequently collecting blood samples for glucose and insulin during an intravenous glucose tolerance test, and analyzing the glucose and insulin dynamics using a minimal mathematical model developed by Bergman (Bergman et. al., J. Clin. Invest. 68:1456-1467 (1981)).
- This test can be modified by the injection of tolbutamide or exogenous insulin to boost the insulin response and improve the correlation with the euglycemic clamp.
- the FSIGTT provides an accessible way to determine the insulin sensitivity index (S 1 ). The more insulin insensitive a subject is, the lower the calculated S 1 . As insulin sensitivity is improved, the S 1 value is increased—thus a higher S 1 value corresponds to greater insulin sensitivity.
- Improving insulin sensitivity and glucose tolerance will help delay and even prevent the onset of Type 2 diabetes mellitus, and thus improve the quality of life by preventing acute and long-term complications, reducing mortality and treating accompanying disorders of those at risk for Type 2 diabetes.
- the invention relates to methods for selecting a diet and exercise regimen for a patient and to methods for treating a patient comprising selecting a diet and exercise regimen.
- the invention provides a method for selecting a diet and exercise regimen for a patient, comprising conducting a particular physical therapy evaluation and a particular nutritional education evaluation of the patient; and selecting a diet and exercise regimen for the patient based on the results of those two evaluations.
- the invention provides a method for treating a patient, comprising selecting a diet and exercise regimen according to the method described above; prescribing the selected diet and exercise regimen to the patient; monitoring the results achieved by the patient; and modifying the diet and/or exercise regimen based on those results.
- FIG. 1 shows a Nutrition Education and Physical Therapy Floxy Chart that may be used in conjunction with the methods of the present invention disclosed herein.
- FIG. 2 shows a Screening Protocol for School Children that may be used to identify patients who might benefit from the methods of the present invention disclosed herein.
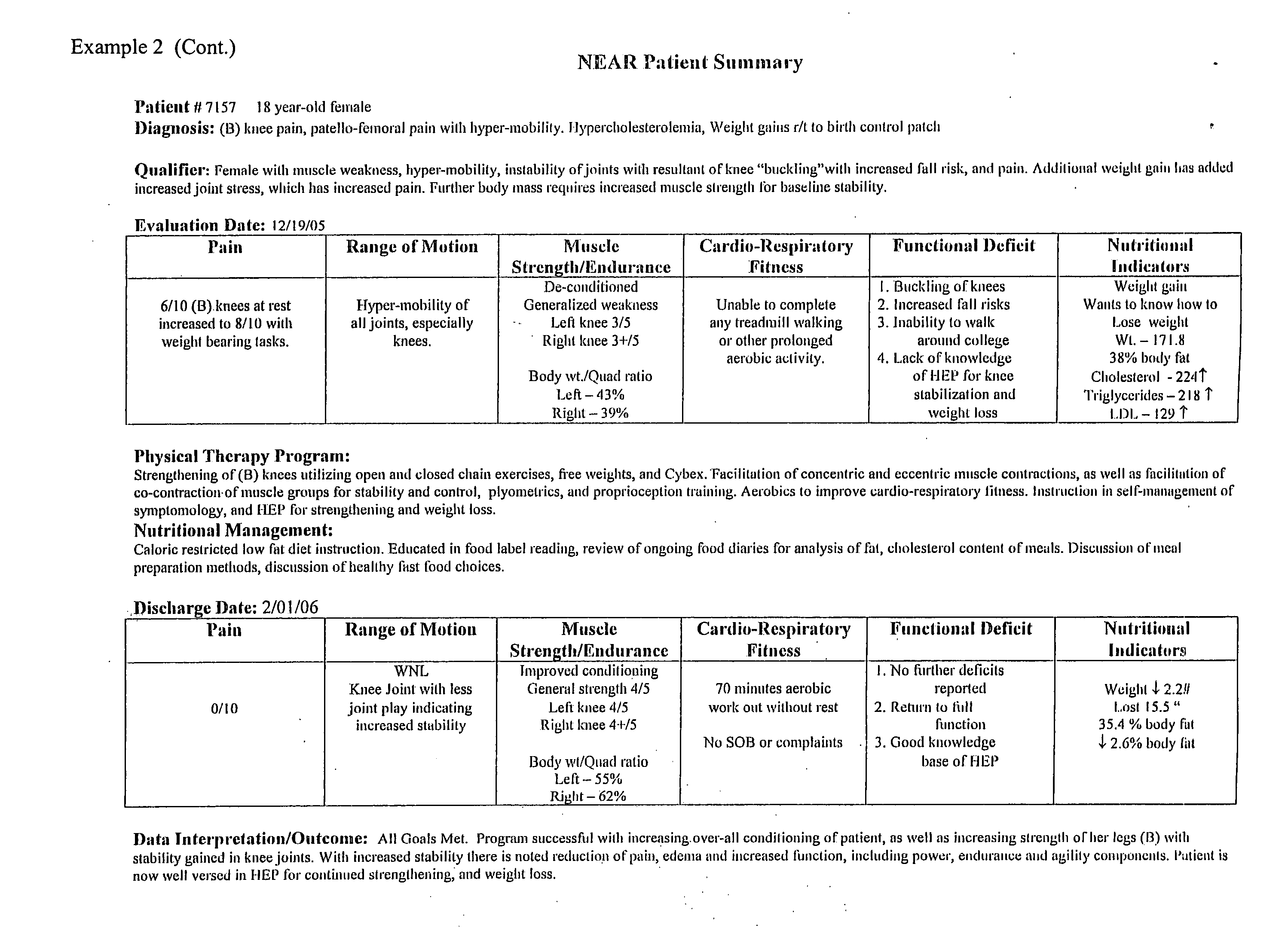
- FIG. 3 shows results achieved using the methods of the present invention.
- FIG. 1 shows a flowchart for evaluating a general patient on first visit.
- FIG. 2 shows a screening protocol for evaluating a juvenile patient for possible type 2 diabetes or a precursor condition thereof, such as IFG, IGT and/or insulin resistant metabolic syndrome.
- a physical therapy evaluation of the patient is performed at the outset of treatment in order to establish “baseline” standards for the various data points. Based, at least in part of the results of that evaluation, a particularized exercise regiment is devised for that patient.
- An initial physical therapy evaluation should cover a wide range of potential causes for concern during treatment, including, but not necessarily limited to, the following: (i) pain issues/concerns; (ii) range of motion; (iii) flexibility; (iv) muscle strength; (v) muscle endurance; (vi) sensory issues; (vii) functional deficits; and (viii) proprioception/balance/fall risk/coordination assessment and cardio-respiratory level.
- the patient will be subject to further such evaluations as described above.
- follow-up evaluations allow the particular exercise regimen for that patient to be adjusted and controlled, as well as allowing the caregiver to monitor the patient's progress towards his/her goals in the program and to adjust the exercise regiment accordingly.
- Such a grid may be used during the initial Physical Therapy Evaluation (to establish baseline values) and throughout the period of treatment, either continuously or periodically, to monitor the results achieved with a given patient and suggest modifications and additions to the exercise regimen.
- Additional factors which may be considered in conjunction with the physical therapy evaluation, particularly based on the Fitness Performance Measurement Grid include, but are not limited to, the following: blood pressure, both at rest and with physical activity; heart rate, both at rest and with physical activity; blood sugar level and reaction to exercise/activity; patient's knowledge bases of diabetes and/or blood sugar and the relation to exercise/activity; psycho-social issue.
- a nutritional education evaluation is also advantageously conducted.
- Such an evaluation should, at least, be conducted at the outset of treatment to establish “baseline” values for future use and consideration.
- a nutritional evaluation is conducted periodically during the treatment phase and the patient's suggested diet adjusted accordingly.
- Such a nutritional education evaluation includes consideration of:
- the results from the physical therapy and nutritional education evaluations provide the caregiver with the ability to develop a baseline determination of a particular patient's risk profile. This determination is the first step in changing the management of the patient's disease.
- the accurate determination of the patient's risk factors will guide the development of a personalized treatment plan based on physical activity and diet. Such as individual's treatment plan can then be modified over time according to the results of follow-up testing.
- the invention further comprises methods for treating a patient comprising selecting a diet and exercise regimen based on the above-described method of selecting a diet and exercise regimen, monitoring the patient throughout the treatment and modifying the diet and/or exercise regimen as appropriate.
- Diet should include fish at least twice each week or should be supplemented with fish oils or flax seeds. Low glycemic index foods should be chosen. Alcohol should be avoided. Careful control of caloric intake is suggested. Patients should refer to “low”, “moderate” and “high” food from the Glycemic Index Table.
- a smaller amount of food at more frequent intervals is also recommended as increasing meal frequency (but not total caloric intake) decreases cardiovascular risk.
- Simple sugars should be avoided. Diet should include fish at least twice each week or should be supplemented with fish oils or flax seeds. Low glycemic index foods should be chosen. Alcohol should be avoided. Smaller meals and more frequent feeding are recommended. Careful control of caloric intake is suggested. Patients should refer to “low”, “moderate” and “high” food from the Glycemic Index Table.
- Foods for use in the diet regimen of the invention can be divided into food categories and subcategories as “healthy, not-as-healthy and splurge” foods.
- the following list provides examples.
- GRAIN & STARCH “healthy” foods are those that are good sources of soluble fiber.
- VEGETABLE “healthy” foods are the most nutrient packed vegetables having three or more of Vitamin E, A, C, K or that are high in soluble fiber.
- PROTEIN “healthy” designates those foods that are categorized by the food exchange system as very lean and includes salmon and soy products because of their heart-healthy properties.
- the “not-as-healthy” category includes meats/meat-substitutes categorized as lean and the “splurge” foods as medium to high-fat.
- DAIRY nonfat and soy (again for its heart-healthy properties) products are listed as “healthy” whereas higher fat choices are in the other two categories.
- FAT categories are divided relative to the type of major fatty-acid the food supplies: “healthy” are those fats that are primarily made up of monounsaturated fatty acids, or omega-3-fatty acids or that are high in isoflavones, “not-as-healthy” are fats containing mostly polyunsaturated fatty acids and “splurge” are those fats that are made up primarily of saturated fatty acids.
- SWEETS categories are divided relative to simple sugar content and dietary fiber.
- the appropriate amount of exercise to promote these responses is dependent upon gender, age, height relative to weight, and the combined risk level of each individual (Halle M et al. (1999) Int J Sports Med 20:464-9).
- the appropriate duration for most activity is around 20-30 minutes but this can vary depending on the intensity of the activity.
- Long-term exercise training increases exercise capacity, which permits larger individual exercise sessions and a greater acute effect.
- the exercise goal to work towards is 20-30 minutes of continuous physical activity on most days of the week.
- the methods of the invention quantify and qualify how much physical activity and the type of such physical activity is appropriate to reduce risk factors relative to an individual's personal set of demographic, medical, and other risk parameters.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- General Health & Medical Sciences (AREA)
- Medical Informatics (AREA)
- Primary Health Care (AREA)
- Public Health (AREA)
- Nutrition Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biophysics (AREA)
- Physical Education & Sports Medicine (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
Disclosed are methods for improving insulin sensitivity and/or glucose metabolism in individuals with abnormal glucose tolerance. Such methods may be used in treating conditions associated with abnormal glucose tolerance and metabolism in mammals, including insulin resistance, insulin resistant metabolic syndrome, impaired glucose tolerance, impaired fasting glucose, hypertension, hypercholestemia and diabetes, particularly Type 2 diabetes.
Description
- This application claims benefit of U.S. Provisional Application Ser. No. 60/662,368 filed Mar. 17, 2006, whose entire disclosure is incorporated herein by reference.
- The present invention relates to methods for improving insulin sensitivity and/or glucose metabolism in individuals with abnormal glucose tolerance. Such methods may be used in treating conditions associated with abnormal glucose tolerance and metabolism in mammals, including insulin resistance, insulin resistant metabolic syndrome, impaired glucose tolerance, impaired fasting glucose, hypertension, hypercholesterolemia and diabetes, particularly
Type 2 diabetes. - Obesity has now reached epidemic proportions globally, with more than 1 billion adults overweight—and at least 300 million of them clinically obese—and is a major contributor to the global burden of chronic disease and disability. Increased consumption of more energy-dense, nutrient-poor foods with high levels of sugar and saturated fats, combined with reduced physical activity, have led to obesity rates that have risen three-fold or more since 1980 in some areas of North America, the United Kingdom, Eastern Europe, the Middle East, the Pacific Islands, Australasia and China.
- Obesity and overweight pose a major risk for serious diet-related chronic diseases, including
Type 2 diabetes, cardiovascular disease, hypertension and stroke, and certain forms of cancer. The health consequences range from increased risk of premature death, to serious chronic conditions that reduce the overall quality of life; of special concern is the increasing incidence of child obesity. - The rising epidemic of obesity and overweight reflects the profound changes in society and in behavioral patterns of communities over recent decades. While genetics may be important in determining a person's susceptibility to weight gain, energy balance is determined by calorie intake and physical activity. Thus, societal changes and worldwide nutrition transition tend to drive the obesity and overweight epidemic.
- Economic growth, modernization, urbanization and globalization of food markets are just some of the forces that have been suggest as contributing to this epidemic. In particular, as incomes rise and populations become more urban, diets high in complex carbohydrates give way to more varied diets with a higher proportion of fats, saturated fats and sugars. At the same time, large shifts towards less physically demanding work have been observed worldwide. Moves towards less physical activity are also found in the increasing use of automated transport, technology in the home, and more passive leisure pursuits.
- The prevalence of overweight and obesity is commonly assessed by using body mass index (BMI), defined as the weight in kilograms divided by the square of the height in meters (kg/m2). A BMI over 25 kg/m2 is defined as overweight, and a BMI of over 30 kg/m2 as obese. These markers provide common benchmarks for assessment, but the risks of disease in all populations can increase progressively from lower BMI levels.
- Raised BMI is also thought to increase the risks of cancer of the breast, colon, prostrate, endometrium, kidney and gallbladder. Chronic overweight and obesity contribute significantly to osteoarthritis, a major cause of disability in adults. Although obesity should be considered a disease in its own right, it is also one of the key risk factors for other chronic diseases together with smoking, high blood pressure and high blood cholesterol. In the analyses carried out for World Health Report 2002, approximately 58% of diabetes and 21% of ischemic heart disease and 8-42% of certain cancers globally were attributable to a BMI above 21 kg/m2.
- Adult mean BMI levels of 22-23 kg/m2 are found in Africa and Asia, while levels of 25-27 kg/m2 are prevalent across North America, Europe, and in some Latin American, North African and Pacific Island countries. BMI increases amongst middle-aged elderly people, who are at the greatest risk of health complications. In countries undergoing nutrition transition, over nutrition often co-exists with undernutrition.
- The distribution of BMI is shifting upwards in many populations. And recent studies have shown that people who were undernourished in early life and then become obese in adulthood, tend to develop conditions such as high blood pressure, heart disease and diabetes at an earlier age and in more severe form than those who were never undernourished.
- Currently more than 1 billion adults are overweight—and at least 300 million of them are clinically obese. Current obesity levels range from below 5% in China, Japan and certain African nations, to over 75% in urban Samoa. But even in relatively low prevalence countries like China, rates are almost 20% in some cities.
- Childhood obesity is already epidemic in some areas and on the rise in others. An estimated 22 million children under five are estimated to be overweight worldwide. According to the US Surgeon General, in the USA the number of overweight children has doubled and the number of overweight adolescents has trebled since 1980. The prevalence of obese children aged 6-11 years has more than doubled since the 1960s. Obesity prevalence in juveniles aged 12-17 has increased dramatically from 5% to 13% in boys and from 5% to 9% in girls between 1966-70 and 1988-91 in the USA. The problem is global and increasingly extends into the developing world; for example, in Thailand the prevalence of obesity in 5-to-12 year olds children rose froml2.2% to 15-6% in just two years.
- Obesity accounts for 2-6% of total health care costs in several developed countries; some estimates put the figure as high as 7%. The true costs are undoubtedly much greater as not all obesity-related conditions are included in the calculations.
- Overweight and obesity lead to adverse metabolic effects on blood pressure, cholesterol, triglycerides and insulin resistance. The non-fatal, but debilitating health problems associated with obesity include respiratory difficulties, chronic musculoskeletal problems, skin problems and infertility. The more life-threatening problems fall into four main areas: CVD problems; conditions associated with insulin resistance such as
type 2 diabetes; certain types of cancers, especially the hormonally related and large-bowel cancers; and gallbladder disease. - The likelihood of developing
Type 2 diabetes and hypertension rises steeply with increasing body fatness. Confined to older adults for most of the 20th century, this disease now affects obese children even before puberty. Approximately 85% of people with diabetes aretype 2, and of these, 90% are obese or overweight. And this is increasingly becoming a developing world problem. In 1995, the Emerging Market Economies had the highest number of diabetics. If current trends continue, India and the Middle Eastern crescent will likely have taken over by 2025. Large increases would likely also be observed in China, Latin America and the Caribbean, and the rest of Asia. - Abnormal glucose tolerance refers to metabolic stages intermediary to normal glucose homeostasis and
Type 2 diabetes; this includes conditions like impaired glucose tolerance (IGT) and impaired fasting glucose (JFG), where glucose values are above the conventional normal range and are often accompanied by a decrease in insulin sensitivity. Impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) are transient, intermediate stages in the development ofType 2 diabetes. Within ten years of diagnosis, approximately 30% of IGT subjects will progress toType 2 diabetes and potentially to health problems that accompany this disease, including retinopathy, nephropathy, and peripheral neuropathy. In addition, abnormal glucose tolerance and decreased insulin sensitivity are associated with a high risk for the development of hypertension, dyslipidemia and an increase incidence of coronary artery disease. - Abnormal glucose tolerance and decreased insulin sensitivity can be attributed to a wide range of causes including obesity, age, physical activity level, certain medication or drugs, genetic factors, and some endocrine related disorders. The truncal distribution of weight as determined by a high waist to hip ratio (WHR) is a good predictor of abnormal insulin sensitivity, and there is an excellent correlation between a high body mass index (BMI) and decreased insulin sensitivity. Approximately 33% of the population in the United States is obese, and the majority of these individuals have decreased insulin sensitivity, are hyperinsulinemic, and often have abnormal glucose tolerance.
- Impaired fasting glucose (IFG) is defined by the American Diabetes Association as a fasting blood glucose in the range of 110 mg/dL to 125 mg/dL. IFG is determined by analysis of a plasma sample for glucose after a 10-16 hour fast. This is an easy and quick way to determine if there is a problem with glucose tolerance and metabolism.
- Impaired glucose tolerance (JGT), as defined by the World Health Organization, is determined by the administration of a standard oral glucose tolerance test (OGT) (World Health Org., Diabetes Mellitus, Tech. Rep. Ser., no. 727 (1985)). During an OGTT, a measured amount of glucose (75 grams) is given to a fasted individual and blood glucose levels are measured every 30 minutes, usually for 2 or 3 hours. In a individual with normal glucose tolerance, the blood glucose values will rise during the first part of the test and then rapidly return to basal levels. In an IGT individual the post prandial glucose levels will rise above the normal range, producing a 2-hour glucose value of 140-199 mg/dL, and return to basal levels at a slow rate.
- Abnormal glucose tolerance is caused in part by inadequate utilization of glucose in the periphery—at the site of the muscles. In addition, high fasting glucose values, seen with impaired fasting glucose, suggest that hepatic glucose production is not being effectively regulated. The underlying cause of this abnormal glucose tolerance is characterized by a decrease in insulin sensitivity.
- Insulin sensitivity is a measurement of insulin's ability to produce a biological response; specifically, in the case of glucose regulation, insulin sensitivity is a measurement of insulin's ability to promote the clearance and utilization of glucose. A decrease in insulin sensitivity will result in a prolonged elevation of glucose levels and the release of additional insulin to try and achieve a euglycemic state. This compensatory hyperinsulinemia will effect insulin's ability to suppress lipolysis in adipose tissue, thus causing an increase in free fatty acids and ultimately resulting in the disruption of normal lipid profiles which could lead to coronary artery disease. The increase in free fatty acids will also inhibit insulin-stimulated glucose utilization in the muscle and stimulate hepatic gluconeogenesis. This leads to increased blood glucose and will eventually result in the development of impaired glucose tolerance or impaired fasting glucose and ultimately, if unchecked, the development of
Type 2 diabetes. Improving insulin sensitivity will restore overall glucose control and decrease the risk of cardiovascular disease. - The level of insulin sensitivity can be measured to varying degrees by three methods: fasting plasma insulin, the euglycemic clamp, and the frequently sampled intravenous glucose tolerance test (FSIGTT). With the use of any of these techniques there is a wide range of insulin sensitivity, with overlapping values characterizing individuals with normal, abnormal glucose tolerance and
Type 2 diabetes. - One method of determining insulin sensitivity is by fasting plasma insulin values. Simply, a high fasting insulin value correlates with decreased insulin sensitivity.
- The euglycemic clamp test procedure involves infusing glucose at a variable rate in order to obtain a constant plasma glucose concentration. This rate of glucose infusion is equal to the overall rate of basal glucose disposal in the body. When insulin is also infused, the glucose infusion rate reflects insulin mediated glucose uptake. This is a precise and reproducible way to determine insulin sensitivity, albeit the euglycemic clamp method is time-consuming and complicated to perform.
- A third method of determining insulin sensitivity involves frequently collecting blood samples for glucose and insulin during an intravenous glucose tolerance test, and analyzing the glucose and insulin dynamics using a minimal mathematical model developed by Bergman (Bergman et. al., J. Clin. Invest. 68:1456-1467 (1981)). This test can be modified by the injection of tolbutamide or exogenous insulin to boost the insulin response and improve the correlation with the euglycemic clamp. The FSIGTT provides an accessible way to determine the insulin sensitivity index (S1). The more insulin insensitive a subject is, the lower the calculated S1. As insulin sensitivity is improved, the S1 value is increased—thus a higher S1 value corresponds to greater insulin sensitivity.
- Improving insulin sensitivity and glucose tolerance will help delay and even prevent the onset of
Type 2 diabetes mellitus, and thus improve the quality of life by preventing acute and long-term complications, reducing mortality and treating accompanying disorders of those at risk forType 2 diabetes. - Accordingly, there remains a need in the art for methods of improving insulin sensitivity and restoring normal glucose tolerance and metabolism in individuals with impaired glucose tolerance, impaired fasting glucose and/or
Type 2 diabetes mellitus. - The invention relates to methods for selecting a diet and exercise regimen for a patient and to methods for treating a patient comprising selecting a diet and exercise regimen.
- In one embodiment, the invention provides a method for selecting a diet and exercise regimen for a patient, comprising conducting a particular physical therapy evaluation and a particular nutritional education evaluation of the patient; and selecting a diet and exercise regimen for the patient based on the results of those two evaluations.
- In yet a further embodiment, the invention provides a method for treating a patient, comprising selecting a diet and exercise regimen according to the method described above; prescribing the selected diet and exercise regimen to the patient; monitoring the results achieved by the patient; and modifying the diet and/or exercise regimen based on those results.
-
FIG. 1 shows a Nutrition Education and Physical Therapy Floxy Chart that may be used in conjunction with the methods of the present invention disclosed herein. -
FIG. 2 shows a Screening Protocol for School Children that may be used to identify patients who might benefit from the methods of the present invention disclosed herein. -
FIG. 3 shows results achieved using the methods of the present invention. - The invention includes methods for selecting an improved or optimal diet and exercise regimen for a patient based on the consideration of several factors, both physical and nutritional.
FIG. 1 shows a flowchart for evaluating a general patient on first visit.FIG. 2 shows a screening protocol for evaluating a juvenile patient forpossible type 2 diabetes or a precursor condition thereof, such as IFG, IGT and/or insulin resistant metabolic syndrome. - In one aspect of the present invention, a physical therapy evaluation of the patient is performed at the outset of treatment in order to establish “baseline” standards for the various data points. Based, at least in part of the results of that evaluation, a particularized exercise regiment is devised for that patient.
- An initial physical therapy evaluation should cover a wide range of potential causes for concern during treatment, including, but not necessarily limited to, the following: (i) pain issues/concerns; (ii) range of motion; (iii) flexibility; (iv) muscle strength; (v) muscle endurance; (vi) sensory issues; (vii) functional deficits; and (viii) proprioception/balance/fall risk/coordination assessment and cardio-respiratory level.
- Periodically during the course of treatment under the inventive method, the patient will be subject to further such evaluations as described above. Such follow-up evaluations allow the particular exercise regimen for that patient to be adjusted and controlled, as well as allowing the caregiver to monitor the patient's progress towards his/her goals in the program and to adjust the exercise regiment accordingly.
-
- The use of the above Fitness Performance Measurement Grid is particularly advantageous with the methods of the present invention. Such a grid may be used during the initial Physical Therapy Evaluation (to establish baseline values) and throughout the period of treatment, either continuously or periodically, to monitor the results achieved with a given patient and suggest modifications and additions to the exercise regimen.
- Additional factors which may be considered in conjunction with the physical therapy evaluation, particularly based on the Fitness Performance Measurement Grid, include, but are not limited to, the following: blood pressure, both at rest and with physical activity; heart rate, both at rest and with physical activity; blood sugar level and reaction to exercise/activity; patient's knowledge bases of diabetes and/or blood sugar and the relation to exercise/activity; psycho-social issue.
- In is advantageous in certain embodiments of the invention to ascertain detailed physical data regarding a patient's body composition, for example by taking weight and size measurements as certain points of the body, for example abdominal thickness and skin fold caliper results. A suitable individual weight and measurement evaluation is shown below:
- In addition to the above physical therapy evaluation and individual weight and measurement, a nutritional education evaluation is also advantageously conducted. Such an evaluation should, at least, be conducted at the outset of treatment to establish “baseline” values for future use and consideration. Preferably, a nutritional evaluation is conducted periodically during the treatment phase and the patient's suggested diet adjusted accordingly.
- Such a nutritional education evaluation includes consideration of:
- (i) Diet History
- (a) assess for intake adequacy;
- (b) review for vitamin and/or mineral deficiencies;
- (c) review for caloric and/or carbohydrate abuse;
- (d) review for food group avoidance;
- (e) evaluate possible of eating disorder, e.g. anorexia, bulimia; and
- (f) review current medications
- (ii) Medical History
- (a) risks of co-morbidity, e.g. sleep apnea, elevated cholesterol, hypertension, ADHD;
- (b) abnormal laboratory values in body fluids (blood, urine, etc);
- (c) possible food/drug interactions—dietary precautions;
- (d) biometrics/anthropometrics;
- (e) basal energy expenditure; and
- (f) percentile placement on growth chart;
- (iii) Social Implications
- (a) family medical history (particularly diabetes, hypertension, hypercholesterolemia, coronary artery disease, alcoholism, depression, drug abuse, smoking, gestational diabetes);
- (b) eating habits particularly fast food, skip meal, frequent eating out); and
- (c) if juvenile, child's history (particularly latch-key, school breakfast and/or lunch programs, etc).
- (iv) Socioeconomic
- (a) number of family members;
- (b) food stamps; and
- (c) low or fixed income.
- (v) Motivation Level/Education Limitations
- (a) reading difficulty;
- (b) comprehension challenges;
- (c) visual learner; and
- (d) inability to read/write.
- Consideration of a plurality of the above factors from each of the physical therapy evaluation, the individual weight and measurement, and the nutritional educational evaluation, and particularly of those factors in combination, may advantageously be used in selecting an improved or optimal diet and exercise regimen according to the methods of the invention for a particular patient.
- More specifically, the results from the physical therapy and nutritional education evaluations provide the caregiver with the ability to develop a baseline determination of a particular patient's risk profile. This determination is the first step in changing the management of the patient's disease. The accurate determination of the patient's risk factors will guide the development of a personalized treatment plan based on physical activity and diet. Such as individual's treatment plan can then be modified over time according to the results of follow-up testing.
- Moreover, the invention further comprises methods for treating a patient comprising selecting a diet and exercise regimen based on the above-described method of selecting a diet and exercise regimen, monitoring the patient throughout the treatment and modifying the diet and/or exercise regimen as appropriate.
- Patients with elevated glucose levels (e.g., 110 mg/dL or higher) should be monitored closely in order to prevent further disease progression and additive complications such as Type-2 diabetes (Packard C et al. (2000) Int J Cardiol 74 Suppl 1:S17-22). Calorie content of preventive diet must be appropriate for weight maintenance or weight loss depending on calculated goal weight or % body fat. Diet should be high in soluble fiber (oat bran, fruits, vegetables), whole grains/starches, fish and plant (soy) proteins and low in processed grains/starches (no sucrose or fructose) and animal proteins. Supplementation with Omega-3 fatty acids (fish oil, flax seeds) is suggested (inihane A M et al. (2000) Arterioscler Thromb Vasc Biol 20:1990-7). A smaller amount of food at more frequent intervals is also recommended as increasing meal frequency (but not total caloric intake) decreases cardiovascular risk. Simple sugars should be avoided. Diet should include fish at least twice each week or should be supplemented with fish oils or flax seeds. Low glycemic index foods should be chosen. Alcohol should be avoided. Careful control of caloric intake is suggested. Patients should refer to “low”, “moderate” and “high” food from the Glycemic Index Table.
- Patients with elevated insulin levels (e.g., 12 uU/ml or higher) should be monitored closely in order to prevent further disease progression and additive complications such as Type-2 diabetes. Calorie content of preventive diet must be appropriate for weight maintenance or weight loss depending on calculated goal weight or % body fat. Diet should be high in soluble fiber (oat bran, fruits, vegetables), whole grains/starches, fish and plant (soy) proteins and low in processed grains/starches (no sucrose or fructose) and animal proteins. Supplementation with Omega-3 fatty acids (fish oil, flax seed) is suggested. A smaller amount of food at more frequent intervals is also recommended as increasing meal frequency (but not total caloric intake) decreases cardiovascular risk. Simple sugars should be avoided. Diet should include fish at least twice each week or should be supplemented with fish oils or flax seeds. Low glycemic index foods should be chosen. Alcohol should be avoided. Careful control of caloric intake is suggested. Patients should refer to “low”, “moderate” and “high” food from the Glycemic Index Table.
- The population of patients with diabetes mellitus represents a very large percentage of patients with high cardiovascular risk (Superko H R (1999) Curr Atheroscler Rep 1:50-7). It is very likely that traditional treatment of these patients actually worsened their condition. Calorie content of preventive diet must be appropriate for weight maintenance or weight loss depending on calculated goal weight or % body fat. Diet should be high in soluble fiber (oat bran, fruits, vegetables), whole grains/starches, fish and plant (soy) proteins and low in processed grains/starches (no sucrose or fructose) and animal proteins. Supplementation with Omega-3 fatty acids (fish oil, flax seed) is suggested. A smaller amount of food at more frequent intervals is also recommended as increasing meal frequency (but not total caloric intake) decreases cardiovascular risk. Simple sugars should be avoided. Diet should include fish at least twice each week or should be supplemented with fish oils or flax seeds. Low glycemic index foods should be chosen. Alcohol should be avoided. Smaller meals and more frequent feeding are recommended. Careful control of caloric intake is suggested. Patients should refer to “low”, “moderate” and “high” food from the Glycemic Index Table.
- The following examples are presented for illustrative purposes only and are not intended, nor should they be construed, as limiting the invention in any way. Those skilled in the art will recognize that variations on the following can be made without exceeding the spirit or scope of the invention.
- Foods for use in the diet regimen of the invention can be divided into food categories and subcategories as “healthy, not-as-healthy and splurge” foods. The following list provides examples.
- GRAIN & STARCH “healthy” foods are those that are good sources of soluble fiber.
- VEGETABLE “healthy” foods are the most nutrient packed vegetables having three or more of Vitamin E, A, C, K or that are high in soluble fiber.
- PROTEIN “healthy” designates those foods that are categorized by the food exchange system as very lean and includes salmon and soy products because of their heart-healthy properties. The “not-as-healthy” category includes meats/meat-substitutes categorized as lean and the “splurge” foods as medium to high-fat.
- DAIRY nonfat and soy (again for its heart-healthy properties) products are listed as “healthy” whereas higher fat choices are in the other two categories.
- FAT categories are divided relative to the type of major fatty-acid the food supplies: “healthy” are those fats that are primarily made up of monounsaturated fatty acids, or omega-3-fatty acids or that are high in isoflavones, “not-as-healthy” are fats containing mostly polyunsaturated fatty acids and “splurge” are those fats that are made up primarily of saturated fatty acids.
- SWEETS categories are divided relative to simple sugar content and dietary fiber.
- Physical activity can positively influence plasma lipid and lipoprotein concentration and reduce coronary artery disease risk levels (Caso E K (1950) Journal of the American Diabetic Association 26:575-583). In summary, physical activity and weight loss have been shown to decrease VLDL, IDL, LDL, APO B, triglycerides, HDL 3, insulin, blood glucose, fibrinogen levels as well as reverse non-insulin dependent diabetes, improve LDL subclass distribution, fibrinolysis and blood pressure and increase LDL particle size, insulin sensitivity and
HDL 2 concentrations (Durstine J L and Thompson P D (2001) Cardiol Clin 19:471-88; Haskell W L (1994) Med Sci Sports Exerc 26:649-660). - The appropriate amount of exercise to promote these responses is dependent upon gender, age, height relative to weight, and the combined risk level of each individual (Halle M et al. (1999) Int J Sports Med 20:464-9). The appropriate duration for most activity is around 20-30 minutes but this can vary depending on the intensity of the activity. For reducing cardiovascular risk levels, it is more important that physical activity is performed on a regular basis as the short-term (acute) effects of exercise that last up to 72 hours greatly improve patients' metabolic condition. Long-term exercise training increases exercise capacity, which permits larger individual exercise sessions and a greater acute effect. The exercise goal to work towards is 20-30 minutes of continuous physical activity on most days of the week.
-
- All of the articles, books, patents, patent applications, and other references cited in this patent application are hereby incorporated by reference.
- Although certain presently preferred embodiments of the invention have been described herein, it will be apparent to those of skill in the art to which the invention pertains that variations and modifications of the described embodiment may be made without departing from the spirit and scope of the invention. Accordingly, it is intended that the invention be limited only to the extent required by the following claims and the applicable rules of law.
Claims (20)
1. A method for determining a diet and exercise regimen for a patient in need thereof, comprising conducting a particular physical therapy evaluation and a particular nutritional education evaluation of the patient; and selecting a diet and exercise regimen for the patient based on the results of those two evaluations.
2. The method according to claim 1 , wherein said physical therapy evaluation comprises: (a) a range of motion/flexibility evaluation; (b) a muscle strength/endurance evaluation; and (c) a cardio-respiratory fitness evaluation.
3. The method according to claim 2 , wherein said physical therapy evaluation further comprises (d) a body composition evaluation.
4. The method according to claim 2 , wherein said range of motion/flexibility evaluation comprises determining range of motion/flexibility for one or more of: (a) sit and reach; (b) pectorals; (c) hamstrings; (d) tensor fascia latae; and (e) hip flexors.
5. The method according to claim 2 , wherein said muscle strength/endurance evaluation comprises determining one or more of: (a) left strength; (b) right grip strength; (c) number of sit-ups in 60 seconds; (d) maximum number of push-ups; (e) one repetition maximum bench press; and (f) one repetition maximum leg press.
6. The method according to claim 2 , wherein said cardio-respiratory fitness evaluation comprises walking on a treadmill for 3 minutes at one or more of: (a) 1.5 mph, no incline; (b) 1.7 mph, 5 degrees incline; (c) 2.5 mph, 7 degrees incline; (d) 3.0 mph, 9 degrees incline; and (e) 1.7 mph, 0 degrees incline.
7. The method according to claim 2 , wherein said physical therapy evaluation further comprises determining one or more of said patient's: resting heart rate; heart rate with physical activity; blood sugar level testing; blood sugar level reaction to physical activity; resting blood pressure; and blood pressure with physical activity.
8. The method according to claim 3 , wherein said body composition evaluation comprises determining one of more of said patient's: height; weight; neck diameter; shoulder width; chest diameter; abdomen diameter; waist diameter; hip diameter; left bicep diameter; right bicep diameter; left forearm diameter; and right forearm diameter.
9. The method according to claim 8 , wherein said body composition evaluation further comprises skin fold measurements at one or more of said patient's: left thigh; left knee; left calf; right thigh; right knee; and right calf.
10. The method according to claim 2 , wherein said nutritional education evaluation comprises consideration and evaluation of one or more of: (a) diet history; (b) medical history; (c) social implications; (d) socioeconomic factors; and (e) motivation level/education limitations.
11. The method according to claim 10 , wherein said consideration and evaluation of diet history comprises assessing one or more of: (a) intake adequacy; (b) vitamin and/or mineral deficiencies; (c) caloric and/or carbohydrate abuse; (d) food group avoidance; (e) possibility of eating disorder; and (f) effect of current medications.
12. The method according to claim 10 , wherein said consideration and evaluation of medical history comprises assessing one or more of: (a) risks of co-morbidity; (b) abnormal laboratory values in body fluids; (c) possible food/drug interactions; (d) biometrics/anthropometrics; (e) basal energy expenditure; and (f) percentile placement on growth chart.
13. The method according to claim 10 , wherein said consideration and evaluation of social implications comprises assessing one or more of the following: (a) family medical history; (b) eating habits; and (c) if said patient is juvenile, said patient's personal circumstances for presence of latch-key, school breakfast and/or lunch programs.
14. The method according to claim 13 , wherein said assessing of family medical history comprises reviewing for the presence of one or more of: diabetes; hypertension; hypercholesterolemia; coronary artery disease; alcoholism; depression; drug abuse; smoking; and/or gestational diabetes.
15. The method according to claim 10 , wherein said consideration and evaluation of socioeconomic factors comprises assessing one or more of the following: (a) number of family members; (b) food stamps; and (c) low or fixed income.
16. The method according to claim 10 , wherein said consideration and evaluation of motivation level/education limitations comprises assessing one or more of the following: (a) reading difficulty; (b) comprehension challenges; (c) visual learner; and (d) inability to read/write.
17. A method for treating a patient suffering from conditions associated with abnormal glucose tolerance and metabolism, comprising selecting a diet and exercise regimen according to the method of claim 1; prescribing the selected diet and exercise regimen to the patient; monitoring the results achieved by the patient; and modifying the diet and/or exercise regimen based on those results.
18. The method according to claim 17 , wherein said patient is suffering from insulin resistance, insulin resistant metabolic syndrome, impaired glucose tolerance, impaired fasting glucose, hypertension, hypercholestemia and/or diabetes.
19. The method according to claim 17 , wherein said patient has a blood glucose level of 110 mg/dl or higher.
20. The method according to claim 17 , wherein said patient has an insulin level of 12 μU/ml or higher.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/377,179 US20060235282A1 (en) | 2005-03-17 | 2006-03-17 | Methods for the treatment of mammals with abnormal glucose tolerance |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US66236805P | 2005-03-17 | 2005-03-17 | |
| US11/377,179 US20060235282A1 (en) | 2005-03-17 | 2006-03-17 | Methods for the treatment of mammals with abnormal glucose tolerance |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20060235282A1 true US20060235282A1 (en) | 2006-10-19 |
Family
ID=37109443
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/377,179 Abandoned US20060235282A1 (en) | 2005-03-17 | 2006-03-17 | Methods for the treatment of mammals with abnormal glucose tolerance |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US20060235282A1 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100074978A1 (en) * | 2007-06-01 | 2010-03-25 | Gela Sulaberidze | Method of treatment and prevention of metabolic and digestion disorders and of pathological states associated therewith and products used therein |
| CN107095657A (en) * | 2017-03-01 | 2017-08-29 | 林万佳 | Metabolism type detection method and metabolism restoration nutrition formula |
| CN109480853A (en) * | 2018-12-26 | 2019-03-19 | 冯鑫 | A kind of automatic adjustable tester of body procurvation in sitting posture |
Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4464122A (en) * | 1982-12-02 | 1984-08-07 | Berkeley Fuller | Health potential summary and incentive system |
| US4566461A (en) * | 1983-02-15 | 1986-01-28 | Michael Lubell | Health fitness monitor |
| US4731726A (en) * | 1986-05-19 | 1988-03-15 | Healthware Corporation | Patient-operated glucose monitor and diabetes management system |
| US4951197A (en) * | 1986-05-19 | 1990-08-21 | Amc Of America | Weight loss management system |
| US5660176A (en) * | 1993-12-29 | 1997-08-26 | First Opinion Corporation | Computerized medical diagnostic and treatment advice system |
| US5673691A (en) * | 1991-01-11 | 1997-10-07 | Pics, Inc. | Apparatus to control diet and weight using human behavior modification techniques |
| US5839901A (en) * | 1997-10-01 | 1998-11-24 | Karkanen; Kip M. | Integrated weight loss control method |
| US5921920A (en) * | 1996-12-12 | 1999-07-13 | The Trustees Of The University Of Pennsylvania | Intensive care information graphical display |
| US5937387A (en) * | 1997-04-04 | 1999-08-10 | Real Age, Inc. | System and method for developing and selecting a customized wellness plan |
| US6024699A (en) * | 1998-03-13 | 2000-02-15 | Healthware Corporation | Systems, methods and computer program products for monitoring, diagnosing and treating medical conditions of remotely located patients |
| US6247004B1 (en) * | 1997-08-18 | 2001-06-12 | Nabil W. Moukheibir | Universal computer assisted diagnosis |
| US20010032099A1 (en) * | 1999-12-18 | 2001-10-18 | Joao Raymond Anthony | Apparatus and method for processing and/or for providing healthcare information and/or healthcare-related information |
| US20020019586A1 (en) * | 2000-06-16 | 2002-02-14 | Eric Teller | Apparatus for monitoring health, wellness and fitness |
| US6454705B1 (en) * | 1999-09-21 | 2002-09-24 | Cardiocom | Medical wellness parameters management system, apparatus and method |
| US6458080B1 (en) * | 2000-05-31 | 2002-10-01 | International Business Machines Corporation | Managing parameters effecting the comprehensive health of a user |
| US6537213B2 (en) * | 1999-10-15 | 2003-03-25 | Hemopet | Animal health care, well-being and nutrition |
| US20030108851A1 (en) * | 2001-12-11 | 2003-06-12 | Posa John G. | Visual feedback methods and apparatus for weight loss and other forms of physical improvement |
| US6605038B1 (en) * | 2000-06-16 | 2003-08-12 | Bodymedia, Inc. | System for monitoring health, wellness and fitness |
| US6607483B1 (en) * | 2000-04-05 | 2003-08-19 | Fitness Holdings, Llc | Method and apparatus for health and fitness feedback |
| US20040010420A1 (en) * | 2001-08-30 | 2004-01-15 | Rooks Daniel S | System for developing implementing and monitoring a health management program |
| US6692436B1 (en) * | 2000-04-14 | 2004-02-17 | Computerized Screening, Inc. | Health care information system |
| US20040034289A1 (en) * | 2000-06-16 | 2004-02-19 | Eric Teller | System for monitoring health, wellness and fitness |
-
2006
- 2006-03-17 US US11/377,179 patent/US20060235282A1/en not_active Abandoned
Patent Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4464122A (en) * | 1982-12-02 | 1984-08-07 | Berkeley Fuller | Health potential summary and incentive system |
| US4566461A (en) * | 1983-02-15 | 1986-01-28 | Michael Lubell | Health fitness monitor |
| US4731726A (en) * | 1986-05-19 | 1988-03-15 | Healthware Corporation | Patient-operated glucose monitor and diabetes management system |
| US4951197A (en) * | 1986-05-19 | 1990-08-21 | Amc Of America | Weight loss management system |
| US5673691A (en) * | 1991-01-11 | 1997-10-07 | Pics, Inc. | Apparatus to control diet and weight using human behavior modification techniques |
| US5660176A (en) * | 1993-12-29 | 1997-08-26 | First Opinion Corporation | Computerized medical diagnostic and treatment advice system |
| US5921920A (en) * | 1996-12-12 | 1999-07-13 | The Trustees Of The University Of Pennsylvania | Intensive care information graphical display |
| US5937387A (en) * | 1997-04-04 | 1999-08-10 | Real Age, Inc. | System and method for developing and selecting a customized wellness plan |
| US6247004B1 (en) * | 1997-08-18 | 2001-06-12 | Nabil W. Moukheibir | Universal computer assisted diagnosis |
| US5839901A (en) * | 1997-10-01 | 1998-11-24 | Karkanen; Kip M. | Integrated weight loss control method |
| US6024699A (en) * | 1998-03-13 | 2000-02-15 | Healthware Corporation | Systems, methods and computer program products for monitoring, diagnosing and treating medical conditions of remotely located patients |
| US6454705B1 (en) * | 1999-09-21 | 2002-09-24 | Cardiocom | Medical wellness parameters management system, apparatus and method |
| US6537213B2 (en) * | 1999-10-15 | 2003-03-25 | Hemopet | Animal health care, well-being and nutrition |
| US20010032099A1 (en) * | 1999-12-18 | 2001-10-18 | Joao Raymond Anthony | Apparatus and method for processing and/or for providing healthcare information and/or healthcare-related information |
| US6607483B1 (en) * | 2000-04-05 | 2003-08-19 | Fitness Holdings, Llc | Method and apparatus for health and fitness feedback |
| US6692436B1 (en) * | 2000-04-14 | 2004-02-17 | Computerized Screening, Inc. | Health care information system |
| US6458080B1 (en) * | 2000-05-31 | 2002-10-01 | International Business Machines Corporation | Managing parameters effecting the comprehensive health of a user |
| US6605038B1 (en) * | 2000-06-16 | 2003-08-12 | Bodymedia, Inc. | System for monitoring health, wellness and fitness |
| US20020019586A1 (en) * | 2000-06-16 | 2002-02-14 | Eric Teller | Apparatus for monitoring health, wellness and fitness |
| US20040034289A1 (en) * | 2000-06-16 | 2004-02-19 | Eric Teller | System for monitoring health, wellness and fitness |
| US20040010420A1 (en) * | 2001-08-30 | 2004-01-15 | Rooks Daniel S | System for developing implementing and monitoring a health management program |
| US20030108851A1 (en) * | 2001-12-11 | 2003-06-12 | Posa John G. | Visual feedback methods and apparatus for weight loss and other forms of physical improvement |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100074978A1 (en) * | 2007-06-01 | 2010-03-25 | Gela Sulaberidze | Method of treatment and prevention of metabolic and digestion disorders and of pathological states associated therewith and products used therein |
| US8834941B2 (en) * | 2007-06-01 | 2014-09-16 | Gela Sulaberidze | Method of treatment and prevention of metabolic and digestion disorders and of pathological states associated therewith and products used therein |
| CN107095657A (en) * | 2017-03-01 | 2017-08-29 | 林万佳 | Metabolism type detection method and metabolism restoration nutrition formula |
| CN109480853A (en) * | 2018-12-26 | 2019-03-19 | 冯鑫 | A kind of automatic adjustable tester of body procurvation in sitting posture |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Kraemer et al. | Physiological adaptations to a weight-loss dietary regimen and exercise programs in women | |
| Tan et al. | Dietary compliance and its association with glycemic control among poorly controlled type 2 diabetic outpatients in Hospital Universiti Sains Malaysia. | |
| Geliebter et al. | Obesity-related hormones and metabolic risk factors: a randomized trial of diet plus either strength or aerobic training versus diet alone in overweight participants | |
| Yao et al. | Relative influence of diet and physical activity on cardiovascular risk factors in urban Chinese adults | |
| Moghadasi et al. | Effects of resistance versus endurance training on plasma lipocalin-2 in young men | |
| Chow et al. | Effects of descending or ascending stair exercise on body composition, insulin sensitivity, and inflammatory markers in young Chinese women with obesity: a randomized controlled trial | |
| Allman et al. | Fat metabolism and acute resistance exercise in trained women | |
| Ormsbee et al. | Pre-sleep protein supplementation after an acute bout of evening resistance exercise does not improve next day performance or recovery in resistance trained men | |
| Muros et al. | Results of a 7-week school-based physical activity and nutrition pilot program on health-related parameters in primary school children in southern Spain | |
| Paris et al. | Increasing energy flux to decrease the biological drive toward weight regain after weight loss–A proof-of-concept pilot study | |
| Saeidi et al. | Resistance training, gremlin 1 and macrophage migration inhibitory factor in obese men: a randomised trial | |
| Binkiewicz-Glińska et al. | Obesity prevention in children and adolescents–Current recommendations | |
| Te Velde et al. | Dairy intake from adolescence into adulthood is not associated with being overweight and metabolic syndrome in adulthood: the Amsterdam growth and health longitudinal study | |
| Mahgoub et al. | Glycaemic index of selected staple carbohydrate-rich foods commonly consumed in Botswana | |
| US20060235282A1 (en) | Methods for the treatment of mammals with abnormal glucose tolerance | |
| Lee et al. | Fish oil administration combined with resistance exercise training improves strength, resting metabolic rate, and inflammation in older adults | |
| Nawawi | The effect of low impact and mixed impact aerobic exercise on percentage of body fat | |
| Zahabi et al. | The effects of l-carnitine supplementation during concurrent training on the functional capacities and body composition in obese men | |
| Abedi | Acute effect of concurrent exercise on serum leptin and resistance insulin response in sedentary men | |
| Park et al. | An eight-week randomized intervention study on Korean adults to evaluate the effect of milk intake on obesity | |
| Shishkova et al. | Analysis of body composition in overweight and obese women using bioimpedance (BIA) system | |
| Zahabi | The effects of l-carnitine supplementation during concurrent training on body composition and functional capacities in obese men | |
| Begum et al. | A study of diagnostic parameters in assessment of metabolic syndrome (MetS) among medical students | |
| Taiwo et al. | Physical exercise and glucose tolerance in Nigerian university students | |
| Grout et al. | A multimeal paradigm producing a low glycemic response is associated with modest cognitive benefits relative to a high glycemic response: a randomized, crossover trial in patients with type 2 diabetes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |